ASTM D6889-03(2017)
(Practice)Standard Practice for Fast Screening for Volatile Organic Compounds in Water Using Solid Phase Microextraction (SPME)
Standard Practice for Fast Screening for Volatile Organic Compounds in Water Using Solid Phase Microextraction (SPME)
SIGNIFICANCE AND USE
4.1 This practice provides a general procedure for the SPME of volatile organic compounds from the headspace of an aqueous matrix. Absorbent extraction is used as the initial step in the extraction of organic constituents for the purpose of screening and subsequently estimating the concentration of the volatile organic components found in water samples. This information may then be used to determine whether a sample may be analyzed directly by purge and trap or headspace or will require dilution prior to analysis.
4.2 Typical detection limits that can be achieved using SPME techniques with GC with a FID range from milligrams per litre (mg/L) to micrograms per litre (μg/L). The detection limit, linear concentration range, and sensitivity of this test method for a specific organic compound will depend upon the aqueous matrix, the fiber phase, the sample temperature, sample volume, sample mixing, and the determinative technique employed.
4.3 SPME has the advantage of speed, reproducibility, simplicity, no solvent, small sample size, and automation.
4.3.1 Extraction devices vary from a manual SPME fiber holder to automated commercial devices specifically designed for SPME.
4.3.2 A partial list of volatile organic compounds that can be screened by this practice is shown in Table 1.
SCOPE
1.1 This practice covers a procedure for the screening of trace levels of volatile organic compounds in water samples by headspace solid phase microextraction (SPME) in combination with fast gas chromatography with flame ionization detection.
1.2 The results from this screening procedure are used to estimate analyte concentrations to prevent contamination of purge and trap or headspace analytical systems.
1.3 The compounds of interest must have a greater affinity for the SPME absorbent polymer or adsorbent than the sample matrix or headspace phase in which they reside.
1.4 Not all of the analytes which can be determined by SPME are addressed in this practice. The applicability of the absorbent polymer, adsorbent or combination to extract the compound(s) of interest must be demonstrated before use.
1.5 Where used it is the responsibility of the user to validate the application of SPME to the analytes of interest.
1.6 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.7 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use. For specific hazard statements, see Section 9.
1.8 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: D6889 − 03 (Reapproved 2017)
Standard Practice for
Fast Screening for Volatile Organic Compounds in Water
Using Solid Phase Microextraction (SPME)
This standard is issued under the fixed designation D6889; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
2.1 ASTM Standards:
1.1 This practice covers a procedure for the screening of
D1129 Terminology Relating to Water
trace levels of volatile organic compounds in water samples by
D1193 Specification for Reagent Water
headspace solid phase microextraction (SPME) in combination
D3694 Practices for Preparation of Sample Containers and
with fast gas chromatography with flame ionization detection.
for Preservation of Organic Constituents
1.2 The results from this screening procedure are used to
D3856 Guide for Management Systems in Laboratories
estimate analyte concentrations to prevent contamination of
Engaged in Analysis of Water
purge and trap or headspace analytical systems.
D4210 Practice for Intralaboratory Quality Control Proce-
dures and a Discussion on Reporting Low-Level Data
1.3 The compounds of interest must have a greater affinity
(Withdrawn 2002)
for the SPME absorbent polymer or adsorbent than the sample
D6520 Practice for the Solid Phase Micro Extraction
matrix or headspace phase in which they reside.
(SPME) of Water and its Headspace for the Analysis of
1.4 Not all of the analytes which can be determined by
Volatile and Semi-Volatile Organic Compounds
SPME are addressed in this practice. The applicability of the
absorbent polymer, adsorbent or combination to extract the 3. Summary of Practice
compound(s) of interest must be demonstrated before use.
3.1 This practice employs adsorbent/gas extraction to iso-
late compounds of interest, see Practice D6520. An aqueous
1.5 Where used it is the responsibility of the user to validate
sample is added to a small (2-mL) septum-sealed vial. Salt is
the application of SPME to the analytes of interest.
used to improve analyte recovery. After the addition of a
1.6 The values stated in SI units are to be regarded as
surrogate standard and a short mixing cycle, a SPME fused
standard. No other units of measurement are included in this
silica fiber coated with a thick polymer film is then exposed to
standard.
the aqueous headspace for a few seconds. The fiber is then
desorbed in the heated injection port of a gas chromatography/
1.7 This standard does not purport to address all of the
flame ionization detector (GC/FID) or gas chromatography/
safety concerns, if any, associated with its use. It is the
mass spectrometry (GC-MS) and the resulting analytes chro-
responsibility of the user of this standard to establish appro-
matographed on a short narrow bore capillary column. The
priate safety, health, and environmental practices and deter-
total analysis time is approximately 3 min.
mine the applicability of regulatory limitations prior to use.
For specific hazard statements, see Section 9.
3.2 The concentrations of the volatile organics in the water
1.8 This international standard was developed in accor-
sample are estimated to determine whether the sample may be
dance with internationally recognized principles on standard- analyzed directly or first diluted prior to purge and trap or
ization established in the Decision on Principles for the
headspace analysis.
Development of International Standards, Guides and Recom-
4. Significance and Use
mendations issued by the World Trade Organization Technical
Barriers to Trade (TBT) Committee.
4.1 This practice provides a general procedure for the
SPMEofvolatileorganiccompoundsfromtheheadspaceofan
1 2
This practice is under the jurisdiction ofASTM Committee D19 on Water and For referenced ASTM standards, visit the ASTM website, www.astm.org, or
is the direct responsibility of Subcommittee D19.06 on Methods for Analysis for contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Organic Substances in Water. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved Dec. 15, 2017. Published December 2017. Originally the ASTM website.
approved in 2003. Last previous edition approved in 2011 as D6889 – 03 (2011). The last approved version of this historical standard is referenced on
DOI: 10.1520/D6889-03R17. www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D6889 − 03 (2017)
aqueous matrix.Absorbent extraction is used as the initial step where:
in the extraction of organic constituents for the purpose of
C ,C , and C = concentrations of the analyte in these
L G F
screening and subsequently estimating the concentration of the
phases.
volatile organic components found in water samples. This
5.1.1 Distribution of the analyte among the three phases:
information may then be used to determine whether a sample
C V 5 C V 1C V 1C V (4)
may be analyzed directly by purge and trap or headspace or
0 L G G L L F F
will require dilution prior to analysis.
5.1.2 Concentration of analyte in fiber:
4.2 Typical detection limits that can be achieved using
C 5 C V K K //V 1K V 1K K V (5)
F 0 L 1 2 G 1 L 1 2 F
SPME techniques with GC with a FID range from milligrams
per litre (mg/L) to micrograms per litre (µg/L). The detection
6. Interferences
limit, linear concentration range, and sensitivity of this test
6.1 Reagents, glassware, septa, fiber coatings, and other
method for a specific organic compound will depend upon the
aqueous matrix, the fiber phase, the sample temperature, sample processing hardware may yield discrete artifacts or
elevated baselines that can cause poor precision and accuracy.
sample volume, sample mixing, and the determinative tech-
nique employed. See Terminology D1129.
6.1.1 Plastics other than PTFE-fluorocarbon should be
4.3 SPME has the advantage of speed, reproducibility,
avoided. They are a significant source of interference and can
simplicity, no solvent, small sample size, and automation.
adsorb some organics.
4.3.1 Extraction devices vary from a manual SPME fiber
holder to automated commercial devices specifically designed
7. Apparatus
for SPME.
4.3.2 Apartiallistofvolatileorganiccompoundsthatcanbe
7.1 SPME Holder, manual or automated sampling.
screened by this practice is shown in Table 1.
7.1.1 SPME Fiber Assembly—Polydimethylsiloxane
(PDMS), 30 uM or equivalent fiber suitable for volatiles
5. Principles of SPME
adsorption.
5.1 SPME is an equilibrium technique where analytes are
7.2 Vials with Septa and Caps, for manual or automated
not completely extracted from the matrix.With liquid samples,
SPME. Vials for automation, 2 mL.
the recovery is dependent on the partitioning or equilibrium of
7.3 Gas Chromatograph (GC), with flame ionization detec-
analytes among the three phases present in the sampling vial:
tor.
theaqueoussampleandheadspace(Eq1),thefibercoatingand
7.3.1 GC Column, 10 m by 0.25 mm, 1 uM film methyl
aqueoussample(Eq2),andthefibercoatingandtheheadspace
silicone, or equivalent.
(Eq 3):
7.3.2 GC Guard Column, 1 m by 0.32 mm uncoated, or
K 5 C /C (1)
1 L g
equivalent.
K 5 C /C (2)
2 F L
7.3.3 Split/splitless Injector, with 0.75 to 1.0 mm inside
K 5 C /C (3)
3 F G diameter insert.
7.3.4 Optional Septum Replacement Device.
7.3.5 Optional SPME Autosampler.
7.3.6 GC Compatible Workstation.
TABLE 1 Check Standard Composition for Screening Volatile
Organic Compounds in Water
8. Reagents
Sample
Detection Limit,
Analyte Composition,
8.1 Purity of Water—Unless otherwise indicated, reference
µg/L
µg/L
towatershallbeunderstoodtomeanreagentwaterconforming
TBA 100 000 10 000
to Type II of Specification D1193.
Methyl-t-butyl ether 1000 150
cis-1,2-Dichloroethene 3000 300
8.2 Chemicals, standard materials, and surrogates should be
1,1,1-Trichloroethane 1000 200
reagent orACS grade or better. When they are not available as
Benzene 400 40
1,1,1-Trichloroethane 700 120
reagent grade, they should have an assay of 90 % or better.
Toluene 200 10
Tetrachloroethene 300 50
8.3 Sodium Chloride (NaCl), reagent grade, granular.
Chlorobenzene 150 10
Ethylbenzene 100 5
8.4 Surrogate Standard, 30 mg/L, 1,4-dichlorobenzene-d
m-Xylene 100 5
in methanol.
styrene 100 5
o-Xylene 100 5
8.5 Check Standard—Prepare a check standard in methanol.
Isopropylbenzene 100 5
Check standard should contain 30-mg/L 1,4-
2-Chlorotoluene 100 5
1,2,4-Trimethylbenzene 100 5 dichlorobenzene-d plus volatile organic compounds that will
1,4-Dichlorobenzene-d4 150 5
be screened. A typical check standard will provide aqueous
1,2-Dichlorobenzene 100 5
concentrations shown in Table 1 when spiking 4 µL of check
Napthalene 100 5
standard to 700-µL water sample.
D6889 − 03 (2017)
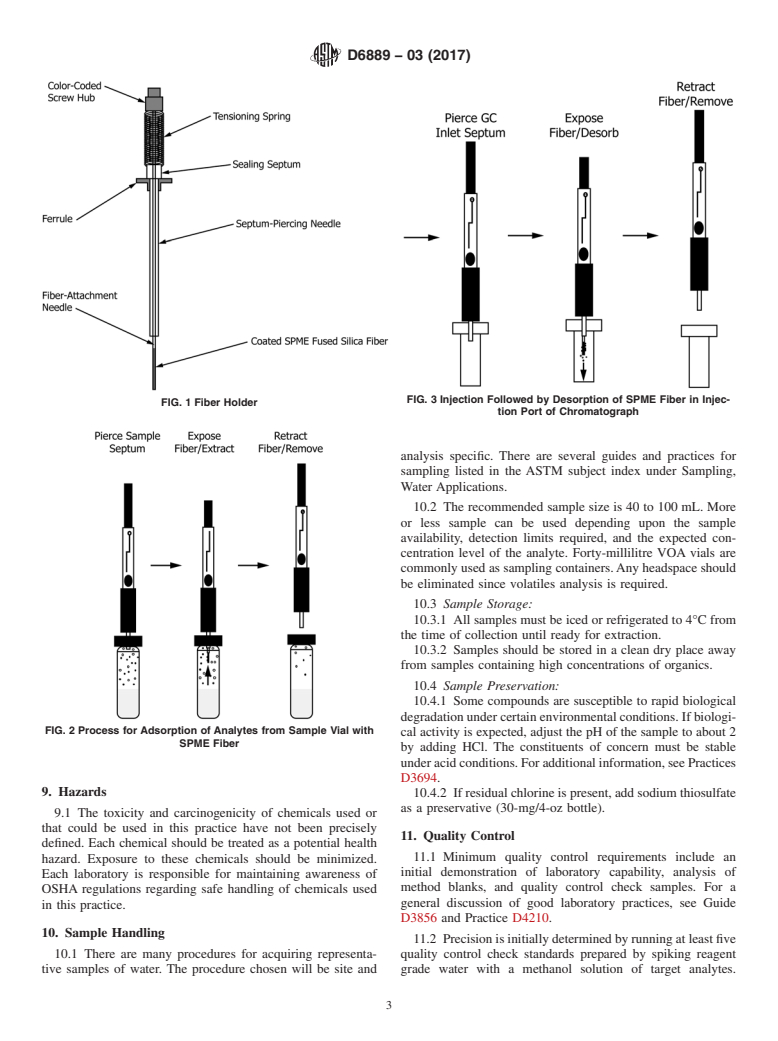
FIG. 3 Injection Followed by Desorption of SPME Fiber in Injec-
FIG. 1 Fiber Holder
tion Port of Chromatograph
analysis specific. There are several guides and practices for
sampling listed in the ASTM subject index under Sampling,
Water Applications.
10.2 The recommended sample size is 40 to 100 mL. More
or less sample can be used depending upon the sample
availability, detection limits required, and the expected con-
centration level of the analyte. Forty-millilitre VOA vials are
commonly used as sampling containers.Any headspace should
be eliminated since volatiles analysis is required.
10.3 Sample Storage:
10.3.1 All samples must be iced or refrigerated to 4°C from
the time of collection until ready for extraction.
10.3.2 Samples should be stored in a clean dry place away
from samples containing high concentrations of organics.
10.4 Sample Preservation:
10.4.1 Some compounds are susceptible to rapid biological
degradation under certain environmental conditions. If b
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.