ASTM F2097-01
(Guide)Standard Guide for Design and Evaluation of Primary Packaging for Medical Products
Standard Guide for Design and Evaluation of Primary Packaging for Medical Products
SCOPE
1.1 This guide provides directions for the design and evaluation of primary packages for medical products. The package materials must be selected appropriately for manufacturing process, end use, and the product being packaged.
1.2 This guide provides a compendium of test methods. Specific individual test methods must be selected based on the pertinent characteristics of the specific product to be packaged and the purpose for testing, research and development, or compliance. Not all test methods will be applicable.
1.3 This guide does not address acceptability criteria, which need to be determined jointly by the package producer and the medical products manufacturer.
1.4 This guide does not assess the product to be packaged; the sterilization method to be used; or package performance through sterilization, distribution, and handling.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:F2097–01
Standard Guide for
Design and Evaluation of Primary Packaging for Medical
Products
This standard is issued under the fixed designation F 2097; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope D 1777 Test Method for Thickness of Textile Materials
D 1894 Test Method for Static and Kinetic Coefficients of
1.1 This guide provides directions for the design and evalu-
Friction of Plastic Film and Sheeting
ation of primary packages for medical products. The package
D 1922 Test Method for Propagation Tear Resistance of
materials must be selected appropriately for manufacturing
Plastic Film and Thin Sheeting by Pendulum Method
process, end use, and the product being packaged.
D 1938 Test Method for Tear Propagation Resistance of
1.2 This guide provides a compendium of test methods.
Plastic Film and Thin Sheeting by a Single Tear Method
Specific individual test methods must be selected based on the
D 2019 Test Method for Dirt in Paper and Paperboard
pertinent characteristics of the specific product to be packaged
D 2457 Test Method for Specular Gloss of Plastic Film and
and the purpose for testing, research and development, or
Solid Plastics
compliance. Not all test methods will be applicable.
D 3078 Test Method for Determination of Leaks in Flexible
1.3 This guide does not address acceptability criteria, which
Packaging by Bubble Emission
need to be determined jointly by the package producer and the
D 3335 Test Method for Low Concentrations of Lead,
medical products manufacturer.
Cadmium, and Cobalt in Paint by Atomic Absorption
1.4 This guide does not assess the product to be packaged;
Spectroscopy
the sterilization method to be used; or package performance
D 3420 Test Method for Dynamic Ball Burst Pendulum
through sterilization, distribution, and handling.
Impact Resistance of Plastic Film
2. Referenced Documents D 3718 Test Method for Low Concentrations of Chromium
in Paint by Atomic Absorption Spectroscopy
2.1 ASTM Standards:
D 3776 Test Methods for Mass per Unit Area (Weight) of
D 374 Test Methods for Thickness of Solid Electrical Insu-
Woven Fabric
lation
D 3985 Test Method for Oxygen Gas Transmission Rate
D 589 Test Method for Opacity of Paper (15 % Diffuse
Through Plastic Film and Sheeting Using a Columetric
Illuminant A, 89 % Reflectance Backing and Paper Back-
Sensor
ing)
D 4279 Test Methods for Water Vapor Transmission of
D 645/D 645M Test Method for Thickness of Paper and
Shipping Containers—Constant and Cycle Methods
Paperboard
D 4321 Test Method for Package Yield of Plastic Film
D 726 Test Methods for Resistance of Nonporous Paper to
D 4754 Test Method for Two-Sided Liquid Extraction of
Passage of Air
Plastic Materials Using FDA Migration Cell
D 882 Test Methods for Tensile Properties of Thin Plastic
D 5264 Practice forAbrasion Resistance of Printed Materi-
Sheeting
als by the Sutherland Rub Tester
D 1003 Test Method for Haze and Luminous Transmittance
F 88 Test Method for Seal Strength of Flexible Barrier
of Transparent Plastics (Gardner)
Materials
D 1709 Test Methods for Impact Resistance of Plastic Film
F 151 TestMethodforResidualSolventsinFlexibleBarrier
by Free-Falling Dart Method
Materials
This guide is under the jurisdiction of ASTM Committee F02 on Flexible
Barrier Materials and is the direct responsibility of Subcommittee F02.60 on
Medical Packaging. Annual Book of ASTM Standards, Vol 07.01.
Current edition approved Oct. 10, 2001. Published December 2001. Annual Book of ASTM Standards, Vol 08.02.
2 7
Annual Book of ASTM Standards, Vol 10.01. Annual Book of ASTM Standards, Vol 06.01.
3 8
Annual Book of ASTM Standards, Vol 15.09. Annual Book of ASTM Standards, Vol 07.02.
4 9
Annual Book of ASTM Standards, Vol 08.01. Annual Book of ASTM Standards, Vol 08.03.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
F2097–01
F 372 Test Method for Water Vapor Transmission Rate of 2.5 TAPPI Standards:
Flexible Barrier Materials Using an Infrared Detection TAPPI T 404 Tensile Breaking Strength and Elongation of
Technique Paper and Paperboard
F 392 Test Method for Flex Durability of Flexible Barrier TAPPI T 437 Dirt in Paper and Paperboard
Materials TAPPI T 460 Air Resistance of Paper (Gurley Method)
F 748 Practice for Selecting Generic Biological Test Meth- TAPPI T 494 Tensile Breaking Properties of Paper and
ods for Materials and Devices Paperboard (Using Constant Rate of Elongation Appara-
F 813 PracticeforDirectContactCellCultureEvaluationof tus)
Materials for Medical Devices TAPPI T 536 Resistance of Paper to Passage of Air (High
F 895 Test Method forAgar Diffusion Cell Culture Screen- Pressure Gurley Method)
ing for Cytotoxicity
3. Terminology
F 904 Test Method for Comparison of Bond Strength or Ply
AdhesionofSimilarLaminatesMadefromFlexibleBarrier
3.1 Definitions of Terms Specific to This Standard:
Materials 3.1.1 barrier requirements, n—the need to promote or
F 1140 Test Methods for Failure Resistance of Unrestrained inhibit moisture, gas, or light, or a combination thereof, while
and Nonrigid Packages for Medical Applications
maintaining necessary levels of sterility.
F 1249 Test Method for Water Vapor Transmission Rate 3.1.2 durability requirements, n—material properties rel-
Through Plastic Film and Sheeting Using a Modulated
evant to the ability of the package to protect the product.
Infrared Sensor 3.1.3 integrity and seal requirements, n—the ability of the
F 1306 Test Method for Slow Rate Penetration Resistance
package to prevent inadvertent escape of contents or entrance
of Flexible Barrier Films and Laminates of outside substances while preserving intended opening for
F 1327 TerminologyRelatingtoBarrierMaterialsforMedi-
use features.
cal Packaging 3.1.4 printing requirements, n—the printed ink properties
F 1443 Practice for Using 0.008-in. (0.203-mm) Aperature
needed to ensure physical and chemical resistance to degrada-
Reflectometers as Test Instruments for Measuring Visual tion.
Image Quality of Business Copy Images
3.1.5 processing requirements, n—the material characteris-
F 1608 Test Method for Microbial Ranking of Porous Pack- tics needed to ensure the consistent and reliable production of
aging Materials (Exposure Chamber Method)
the package.
F 1884 Test Method for Determining Residual Solvents in 3.1.6 safety requirements, n—safeguard product against
Packaging Materials
contamination and deleterious health effects.
F 1886 Test Method for Determining Integrity of Seals for 3.1.7 visibility and appearance requirements, n—the de-
Medical Packaging by Visual Inspection
sired package aesthetics needed to permit or inhibit viewing of
F 1929 Test Method for Detecting Seal Leaks in Porous the product or to enhance product presentation.
Packaging by Dye Penetration 3.2 For other terms used in this guide see Terminology
F 1980 Guide for Accelerated Aging of Sterile Medical
F 1327.
Device Packages
4. Significance and Use
F 2054 Test Method for Burst Testing of Flexible Package
Seals using Internal Air Pressurization with Restraining
4.1 This Design and Evaluation guide describes seven
Plates
categories for evaluating medical packages and packaging
F 2096 Test Method for Detecting Gross Leaks in Porous
materials. These include safety, barrier properties, durability,
Medical Packaging by Internal Pressurization (Bubble
package and seal integrity, visibility and appearance, process-
Test)
ing, and printing ink properties.
2.2 EN/ISO Standards:
4.2 The intent of this Design and Evaluation guide is to
EN 868/1 Annex C Gurley, Schopper, Dye Penetration
evaluate all seven categories and select those that are appli-
ISO 5636/5
cable. Once the product has been characterized and the
ISO 11607 Annex A Gurley
sterilizationmethodologyhasbeendefined,therearenumerous
2.3 Military Specification:
sets of requirements for any specific package. This Design and
Mil Spec 36954C Bacterial Filtration Efficiency
Evaluation guide provides an avenue for assessing these
2.4 FPA Standard:
requirementsandchoosingtestmethodsforbothevaluatingthe
FPA/SPMC 009 Standard Test Method for Coating/
package design and monitoring package compliance.
Adhesive Weight Determination
4.3 Product characterization shall include mass or weight,
geometry (length and width, height and shape) and product
composition.
4.4 All seven categories must be considered for applicabil-
Annual Book of ASTM Standards, Vol 13.01.
Available from International Organization for Standardization (ISO) 1 rue de
ity.
Varembé, P.O. Box 56, CH-1211, Geneva 20, Switzerland.
Available from Standardization Documents Order Desk, Bldg. 4 Section D,
700 Robbins Ave., Philadelphia, PA 19111-5094, Attn: NPOPS.
13 14
AvailablefromtheFlexiblePackagingAssociation,971CorporateBlvd.,Suite Available from the TechnicalAssociation of the Pulp and Paper Industry, P.O.
403, Linthicum, MD 21090. Box 105113, Atlanta, GAA 30348.
F2097–01
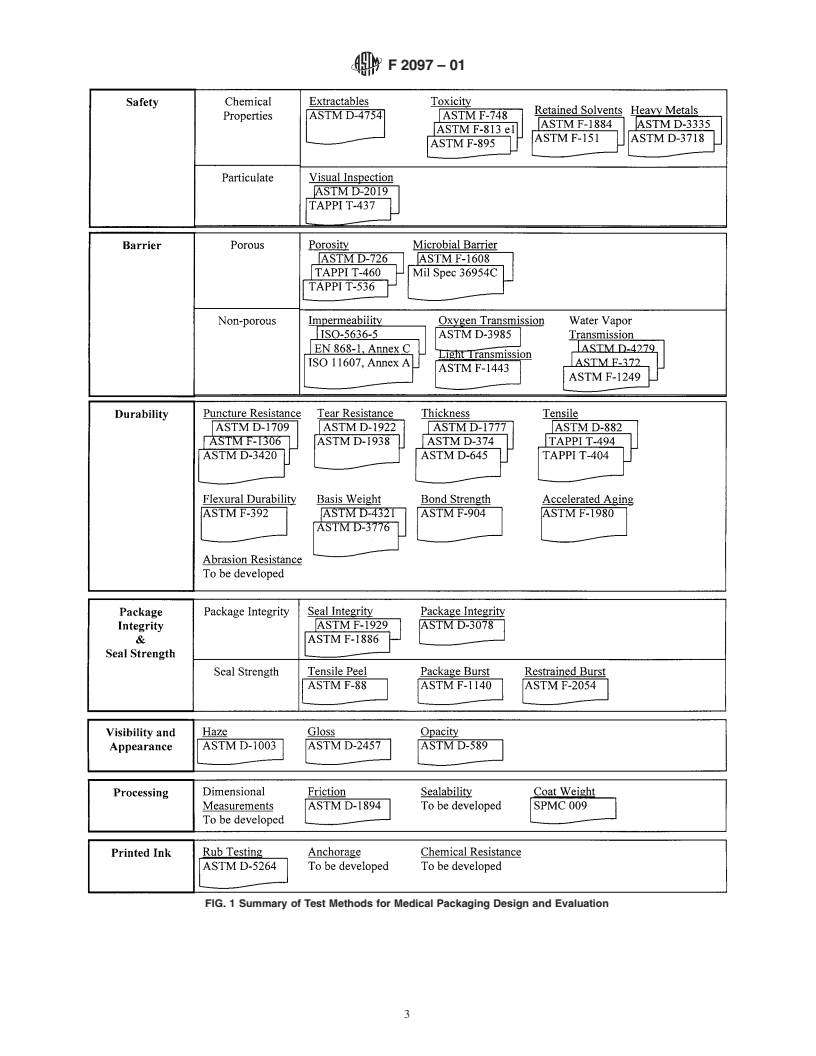
FIG. 1 Summary of Test Methods for Medical Packaging Design and Evaluation
F2097–01
4.5 The Summary of Test Methods for Medical Packaging expensive and require specialized equipment not readily avail-
Design and Evaluation (Fig. 1) provides a compact graphical able at a medical packaging or device manufacturing facility.
presentation of the test methods referenced in this guide.
4.6.1.2 Package Compliance: Routine Monitoring of Adher-
4.6 Test Description and Applicability (see Table 1):
ence to Specifications—This is referred to as “Compliance
4.6.1 Table 1 lists the test methods commonly used to
Testing” in Table 1. Testing during this phase must be rapid,
evaluate medical packaging. The test methods are used in two
inexpensive, and readily implemented in a manufacturing
phases.
environment.Theobjectiveisnottodevelopdesigndata,butto
4.6.1.1 Package Design: Characterization of the Materials
ensure that the design specifications are being met. These test
and Evaluation of the Resultant Package—This is referred to
methods do not necessarily make direct measurements of
as “R&D Evaluation” in Table 1. Testing during this phase is
critical values, but detect variations in material, process, or
characterized by the generation of quantitative data on the
product that are indicative of all critical characteristics.
performance of the component materials and the package
4.6.2 It is important to note that no individual test method is
assembly. These test methods are lengthy, making them inap-
entirely predictive of final package performance. Filled pack-
propriate for the manufacturing environment where rapid
response is required for process control. Often, they are ages must be evaluated under conditions of use.
TABLE 1 Test Description and Applicability Table
Test Test Method Description Applicability
Safety Requirements
Chemical Properties
Extractibles ASTM D 4754 This test method covers the use of the FDA migration This test method has been applied to a variety of
Useage cell in the extraction of components and permits migrant/polymer systems in contact with numerous foods
R&D evaluation quantitation of individual migrants from plastic and food simulants. Though most of the migrants
materials by suitable extracting liquids, including liquid examined were radiolabeled, the use of the FDA cell has
foods and food-stimulating solvents. This test method been validated for migration studies of unlabeled styrene
provides a two-sided, liquid extraction test for plastic from polystyrene.
materials that can be formed into film, sheet, or disks. This test method has been shown to yield reproducible
results under the conditions for migration tests requested
by the FDA. However, if the data is to be submitted to the
FDA, it is suggested that their guidelines by consulted.
Because it employs two-sided extraction, this test method
may not be suitable for multilayered plastics intended for
single-sided food contact use.
The size of the FDA migration cell as described may
preclude its use in determining total nonvolatile
extractives in some cases.
Toxity ASTM F 748 This practice recommends generic biological test methods The biocompatibility of materials used in single-
Useage for materials and devices according to end-use applica- component or multicomponent medical devices for human
R&D evaluation tions. Tests include those performed on materials, end use depends to a large degree on the particular nature of
products, and extracts. Rationale and comments on the end-use application. It is not possible to specify a set
current state of the art are included for all test pro- of biocompatibility test methods which will be necessary
cedures described. Biological evaluation of materials and sufficient to establish biocompatibility for all materials
and devices, and related subjects such as pyrogen and applications.
testing and batch testing of production lots are also While chemical testing for extractable additives and
discussed. residual monomers or residues from processing aids is
necessary for most implant materials, such testing is not
included as a part of this practice. The reader is
cautioned that the area of materials biocompatibility
testing is a rapidly evolving field, and improved methods
are evolving rapidly, so this practice is by necessity only
a guideline. These test protocols are intended to apply to
materials and medical devices for human application.
Toxity ASTM F 813 This practice describes a reference method of direct This practice tends to be used less frequently due to the
Useage contact cell culture testing that may be used in evaluating risk of inducing a response from mechanical damage due
R&D evaluation the cytotoxic potential of materials for use in the con- to direct placement of the sample onto the cell layer. This
struction of medical materials and devices. This practice practice may be suitable for products which have
may be used either directly to evaluate materials or as a leachates that are not able to diffuse through agar and
reference against which other cytotoxicity test methods are not too heavy.
may be compared.
F2097–01
Test Test Method Description Applicability
Toxicity ASTM F 895 The agar diffusion assay is an indirect contact test in This is one of a series of reference test methods for the
Usage which
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.