ASTM F1800-04
(Test Method)Standard Test Method for Cyclic Fatigue Testing of Metal Tibial Tray Components of Total Knee Joint Replacements
Standard Test Method for Cyclic Fatigue Testing of Metal Tibial Tray Components of Total Knee Joint Replacements
SCOPE
1.1 This test method covers a procedure for the fatigue testing of metallic tibial trays used in knee joint replacements. This test method covers the procedures for the performance of fatigue tests on metallic tibial components using a cyclic, constant-amplitude force. It applies to tibial trays which cover both the medial and lateral plateaus of the tibia. This test method may require modifications to accommodate other tibial tray designs.
1.2 This test method is intended to provide useful, consistent, and reproducible information about the fatigue performance of metallic tibial trays with one unsupported condyle.
1.3 The values stated in SI units are regarded as the standard. The inch-pound units given in parentheses are for information only.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:F1800–04
Standard Test Method for
Cyclic Fatigue Testing of Metal Tibial Tray Components of
1
Total Knee Joint Replacements
This standard is issued under the fixed designation F 1800; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 3. Terminology
1.1 This test method covers a procedure for the fatigue 3.1 Definitions:
testing of metallic tibial trays used in knee joint replacements. 3.1.1 R value—TheRvalueistheratiooftheminimumload
This test method covers the procedures for the performance of to the maximum load.
fatigue tests on metallic tibial components using a cyclic,
minimum load
R 5 (1)
constant-amplitude force. It applies to tibial trays which cover
maximum load
both the medial and lateral plateaus of the tibia. This test
3.2 Definitions of Terms Specific to This Standard:
method may require modifications to accommodate other tibial
3.2.1 anteroposterior centerline—a line that passes through
tray designs.
the center of the tibial tray, parallel to the sagittal plane and
1.2 This test method is intended to provide useful, consis-
perpendicular to the line of load application. For asymmetric
tent, and reproducible information about the fatigue perfor-
tibial tray designs, the appropriate center of the tibial tray shall
mance of metallic tibial trays with one unsupported condyle.
be determined by the investigator and the rationale reported.
1.3 The values stated in SI units are regarded as the
3.2.2 fixture centerline—a line that passes through the
standard. The inch-pound units given in parentheses are for
center of the fixture, parallel to the anteroposterior centerline.
information only.
This line represents the separation between the supported and
1.4 This standard does not purport to address all of the
unsupported portions of the test fixture.
safety concerns, if any, associated with its use. It is the
3.2.3 mediolateral centerline—a line that passes through
responsibility of the user of this standard to establish appro-
the center of the tibial tray, parallel to the coronal, or frontal,
priate safety and health practices and determine the applica-
plane and perpendicular to the line of load application. For
bility of regulatory limitations prior to use.
asymmetric tibial tray designs, the appropriate center of the
tibial tray shall be determined by the investigator and the
2. Referenced Documents
rationale reported.
2
2.1 ASTM Standards:
3.2.4 moment arm, d —theperpendiculardistancebetween
ap
E 4 Practices for Force Verification of Testing Machines
the mediolateral centerline of the tibia component and the line
E 467 Practice for Verification of Constant Amplitude Dy-
of load application.
namic Loads in an Axial Load Fatigue Testing System
3.2.5 moment arm, d —theperpendiculardistancebetween
ml
E 468 Practice for the Presentation of Constant Amplitude
the anteroposterior centerline of the tibia component and the
Fatigue Results for Metallic Materials
line of load application.
E 1150 Definitions of Terms Relating to Fatigue
4. Significance and Use
4.1 This test method can be used to describe the effects of
1
This test method is under the jurisdiction ofASTM Committee F04 on Medical
materials, manufacturing, and design variables on the fatigue
and Surgical Materials and Devices and is the direct responsibility of Subcommittee
performance of metallic tibial trays subject to cyclic loading
F04.22 on Arthroplasty.
for relatively large numbers of cycles.
Current edition approved Apr. 1, 2004. Published April 2004. Originally
approved in 1997. Last previous edition approved in 2003 as F 1800 – 97 (2003).
4.2 Theloadingoftibialtraydesigns in vivowill,ingeneral,
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
differ from the loading defined in this test method. The results
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
obtained here cannot be used to directly predict in vivo
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. performance. However, this test method is designed to allow
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
1
---------------------- Page: 1 ----------------------
F1800–04
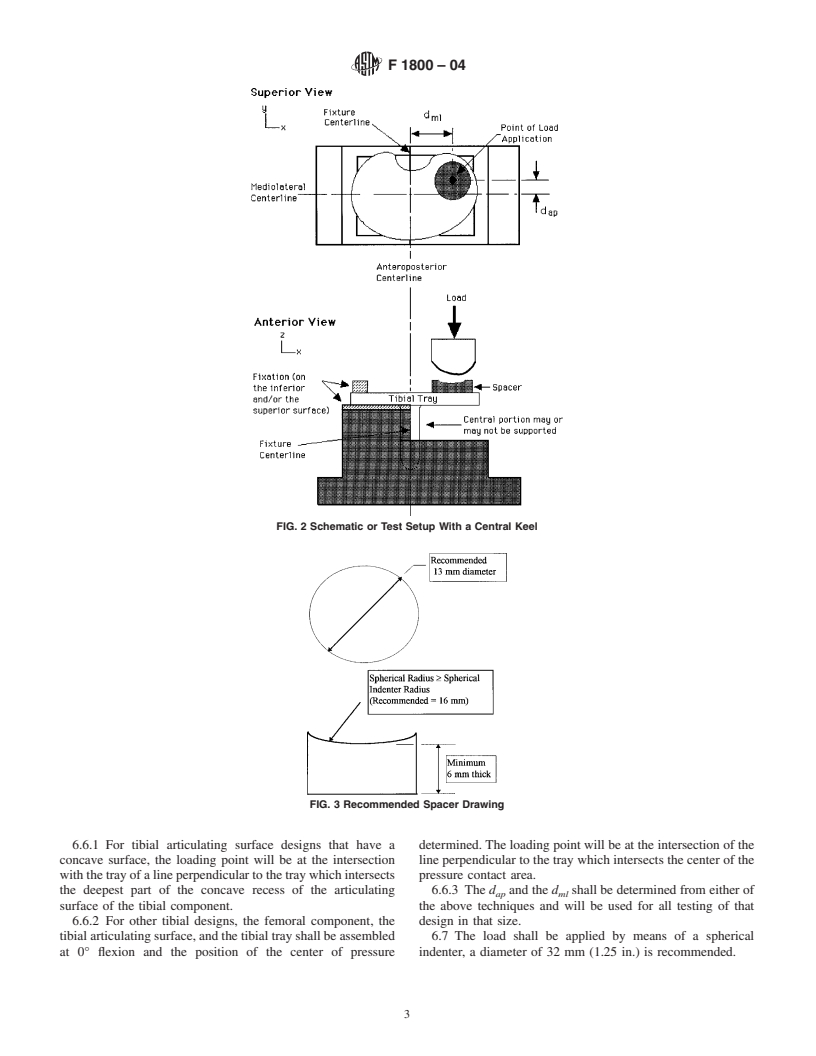
for comparisons between the fatigue performance of different be necessary to evaluate the design with or without support of
metallic tibial tray designs, when tested under similar condi- the keel (see Fig. 2). The method of supporting (or not
tions.
supporting) any such feature shall be reported.
4.3 Inorderforfatiguedataontibia
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.