ASTM F1800-07
(Test Method)Standard Test Method for Cyclic Fatigue Testing of Metal Tibial Tray Components of Total Knee Joint Replacements
Standard Test Method for Cyclic Fatigue Testing of Metal Tibial Tray Components of Total Knee Joint Replacements
SIGNIFICANCE AND USE
This test method can be used to describe the effects of materials, manufacturing, and design variables on the fatigue performance of metallic tibial trays subject to cyclic loading for relatively large numbers of cycles.
The loading of tibial tray designs in vivo will, in general, differ from the loading defined in this test method. The results obtained here cannot be used to directly predict in vivo performance. However, this test method is designed to allow for comparisons between the fatigue performance of different metallic tibial tray designs, when tested under similar conditions.
In order for fatigue data on tibial trays to be comparable, reproducible, and capable of being correlated among laboratories, it is essential that uniform procedures be established.
SCOPE
1.1 This test method covers a procedure for the fatigue testing of metallic tibial trays used in knee joint replacements. This test method covers the procedures for the performance of fatigue tests on metallic tibial components using a cyclic, constant-amplitude force. It applies to tibial trays which cover both the medial and lateral plateaus of the tibia. This test method may require modifications to accommodate other tibial tray designs.
1.2 This test method is intended to provide useful, consistent, and reproducible information about the fatigue performance of metallic tibial trays with one unsupported condyle.
1.3 The values stated in SI units are regarded as the standard. The inch-pound units given in parentheses are for information only.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F1800 − 07
StandardTest Method for
Cyclic Fatigue Testing of Metal Tibial Tray Components of
1
Total Knee Joint Replacements
This standard is issued under the fixed designation F1800; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope E1150 Definitions of Terms Relating to Fatigue (Withdrawn
3
1996)
1.1 This test method covers a procedure for the fatigue
testing of metallic tibial trays used in knee joint replacements.
3. Terminology
This test method covers the procedures for the performance of
3.1 Definitions:
fatigue tests on metallic tibial components using a cyclic,
3.1.1 R value—TheRvalueistheratiooftheminimumload
constant-amplitude force. It applies to tibial trays which cover
to the maximum load.
both the medial and lateral plateaus of the tibia. This test
minimum load
method may require modifications to accommodate other tibial
R 5 (1)
maximum load
tray designs.
3.2 Definitions of Terms Specific to This Standard:
1.2 This test method is intended to provide useful,
3.2.1 anteroposterior centerline—a line that passes through
consistent, and reproducible information about the fatigue
the center of the tibial tray, parallel to the sagittal plane and
performance of metallic tibial trays with one unsupported
perpendicular to the line of load application. For asymmetric
condyle.
tibial tray designs, the appropriate center of the tibial tray shall
be determined by the investigator and the rationale reported.
1.3 The values stated in SI units are regarded as the
standard. The inch-pound units given in parentheses are for
3.2.2 fixture centerline—alinethatpassesthroughthecenter
information only.
of the fixture, parallel to the anteroposterior centerline. This
line represents the separation between the supported and
1.4 This standard does not purport to address all of the
unsupported portions of the test fixture.
safety concerns, if any, associated with its use. It is the
3.2.3 mediolateral centerline—alinethatpassesthroughthe
responsibility of the user of this standard to establish appro-
center of the tibial tray, parallel to the coronal, or frontal, plane
priate safety and health practices and determine the applica-
and perpendicular to the line of load application. For asym-
bility of regulatory limitations prior to use.
metric tibial tray designs, the appropriate center of the tibial
tray shall be determined by the investigator and the rationale
2. Referenced Documents
reported.
2
2.1 ASTM Standards:
3.2.4 moment arm, d —the perpendicular distance between
ap
E4 Practices for Force Verification of Testing Machines
the mediolateral centerline of the tibia component and the line
E467 Practice for Verification of Constant Amplitude Dy-
of load application.
namic Forces in an Axial Fatigue Testing System
3.2.5 moment arm, d —the perpendicular distance between
ml
E468 Practice for Presentation of Constant Amplitude Fa-
the anteroposterior centerline of the tibia component and the
tigue Test Results for Metallic Materials
line of load application.
4. Significance and Use
1
This test method is under the jurisdiction ofASTM Committee F04 on Medical
4.1 This test method can be used to describe the effects of
and Surgical Materials and Devices and is the direct responsibility of Subcommittee
materials, manufacturing, and design variables on the fatigue
F04.22 on Arthroplasty.
Current edition approved Sept. 15, 2007. Published October 2007. Originally performance of metallic tibial trays subject to cyclic loading
approved in 1997. Last previous edition approved in 2004 as F1800 – 04. DOI:
for relatively large numbers of cycles.
10.1520/F1800-07.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
3
Standards volume information, refer to the standard’s Document Summary page on The last approved version of this historical standard is referenced on
the ASTM website. www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F1800 − 07
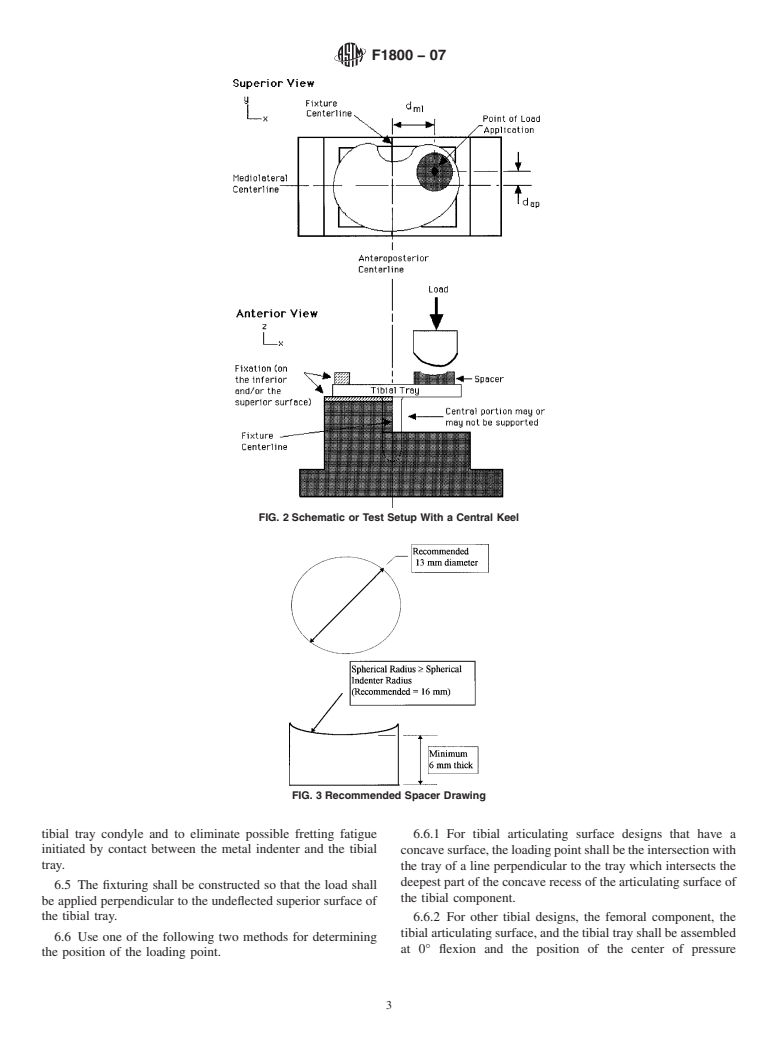
4.2 Theloadingoftibialtraydesigns in vivowill,ingeneral, 6.2 The tibial tray shall be positioned such that the antero-
differ from the loading defined in this test method. The results posterior centerline and the fixture centerline are aligned with
obtained here cannot be used to directly predict in vivo an accuracy of 61mminthe x direction and 62° in the x–y
performance. However, this test method is designed to allow plane (see Fig. 1 and Fig. 2).
fo
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.