ASTM C1662-07
(Practice)Standard Practice for Measurement of the Glass Dissolution Rate Using the Single-Pass Flow-Through Test Method
Standard Practice for Measurement of the Glass Dissolution Rate Using the Single-Pass Flow-Through Test Method
SCOPE
1.1 This practice describes a single-pass flow-through (SPFT) test method that can be used to measure the dissolution rate of a homogeneous silicate glass, including nuclear waste glasses, in various test solutions at temperatures less than 100°C. Tests may be conducted under conditions in which the effects from dissolved species on the dissolution rate are minimized to measure the forward dissolution rate at specific values of temperature and pH, or to measure the dependence of the dissolution rate on the concentrations of various solute species.
1.2 Tests are conducted by pumping solutions in either a continuous or pulsed flow mode through a reaction cell that contains the test specimen. Tests must be conducted at several solution flow rates to evaluate the effect of the flow rate on the glass dissolution rate.
1.3 This practice excludes static test methods in which flow is simulated by manually removing solution from the reaction cell and replacing it with fresh solution.
1.4 Tests may be conducted with demineralized water, chemical solutions (such as pH buffer solutions, simulated groundwater solutions, and brines), or actual groundwater.
1.5 Tests may be conducted with crushed glass of a known size fraction or monolithic specimens having known geometric surface area. The reacted solids may be examined to provide additional information regarding the behavior of the material in the test and the reaction mechanism.
1.6 Tests may be conducted with glasses containing radionuclides. However, this test method does not address safety issues for radioactive samples.
1.7 Data from these tests can be used to determine the values of kinetic model parameters needed to calculate the glass corrosion behavior in a disposal system over long periods (for example, see Practice C 1174).
1.8 This practice must be performed in accordance with all quality assurance requirements for acceptance of the data.
1.9 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
1.10 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:C1662–07
Standard Practice for
For Measurement of the Glass Dissolution Rate Using the
Single-Pass Flow-Through Test Method
This standard is issued under the fixed designation C1662; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 1.9 The values stated in SI units are to be regarded as the
standard. The values given in parentheses are for information
1.1 This practice describes a single-pass flow-through
only.
(SPFT) test method that can be used to measure the dissolution
1.10 This standard does not purport to address all of the
rate of a homogeneous silicate glass, including nuclear waste
safety concerns, if any, associated with its use. It is the
glasses, in various test solutions at temperatures less than
responsibility of the user of this standard to establish appro-
100°C. Tests may be conducted under conditions in which the
priate safety and health practices and determine the applica-
effects from dissolved species on the dissolution rate are
bility of regulatory limitations prior to use.
minimized to measure the forward dissolution rate at specific
values of temperature and pH, or to measure the dependence of
2. Referenced Documents
the dissolution rate on the concentrations of various solute
2.1 ASTM Standards:
species.
C92 Test Methods for SieveAnalysis and Water Content of
1.2 Tests are conducted by pumping solutions in either a
Refractory Materials
continuous or pulsed flow mode through a reaction cell that
C169 Test Methods for Chemical Analysis of Soda-Lime
contains the test specimen. Tests must be conducted at several
and Borosilicate Glass
solution flow rates to evaluate the effect of the flow rate on the
C429 Test Method for Sieve Analysis of Raw Materials for
glass dissolution rate.
Glass Manufacture
1.3 This practice excludes static test methods in which flow
C693 Test Method for Density of Glass by Buoyancy
is simulated by manually removing solution from the reaction
C1109 Practice for Analysis of Aqueous Leachates from
cell and replacing it with fresh solution.
Nuclear Waste Materials Using Inductively Coupled
1.4 Tests may be conducted with demineralized water,
Plasma-Atomic Emission Spectroscopy
chemical solutions (such as pH buffer solutions, simulated
C1174 Practice for Prediction of the Long-Term Behavior
groundwater solutions, and brines), or actual groundwater.
of Materials, Including Waste Forms, Used in Engineered
1.5 Tests may be conducted with crushed glass of a known
Barrier Systems (EBS) for Geological Disposal of High-
size fraction or monolithic specimens having known geometric
Level Radioactive Waste
surface area. The reacted solids may be examined to provide
C1220 Test Method for Static Leaching of Monolithic
additionalinformationregardingthebehaviorofthematerialin
Waste Forms for Disposal of Radioactive Waste
the test and the reaction mechanism.
C1285 Test Methods for Determining Chemical Durability
1.6 Tests may be conducted with glasses containing radio-
of Nuclear, Hazardous, and Mixed Waste Glasses and
nuclides. However, this test method does not address safety
Multiphase Glass Ceramics: The Product Consistency Test
issues for radioactive samples.
(PCT)
1.7 Data from these tests can be used to determine the
D1129 Terminology Relating to Water
values of kinetic model parameters needed to calculate the
D1193 Specification for Reagent Water
glass corrosion behavior in a disposal system over long periods
D1293 Test Methods for pH of Water
(for example, see Practice C1174).
1.8 This practice must be performed in accordance with all
quality assurance requirements for acceptance of the data.
This practice is under the jurisdiction of ASTM Committee C26 on Nuclear
Fuel Cycle and is the direct responsibility of Subcommittee C26.13 on Spent Fuel For referenced ASTM standards, visit the ASTM website, www.astm.org, or
and High Level Waste. contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Current edition approved Feb. 1, 2007. Published March 2007 . DOI: 10.1520/ Standards volume information, refer to the standard’s Document Summary page on
C1662-07. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
C1662–07
E691 Practice for Conducting an Interlaboratory Study to 3.1.20 secondary phase, n—any phase that is not present in
Determine the Precision of a Test Method the glass being tested that is formed in solution or on the
surface of the sample or apparatus by combination of compo-
3. Terminology
nents released from the glass as it dissolved or present in the
3.1
test solution.
3.1.1 alteration phase, n—a solid phase formed as a result
3.1.21 single-pass flow-through test (SPFT), n—a test in
of corrosion, including phases precipitated from solution,
which solution is flushed from the system after contacting the
leached layers, and phases formed within leached layers.
test specimen and is not recirculated through the reaction cell.
3.1.2 back reaction, n—reaction between dissolved compo-
3.1.22 steady-state, adj—in this standard, the condition in
nents and the glass surface to reform bonds that are broken
which the concentration of a dissolved glass component
during glass dissolution.
remains constant due to the opposing effects of solution flow to
3.1.3 chemical durability, n—the resistance of a glass to
remove the components from the vicinity of the sample and
dissolution under particular test conditions.
glass dissolution to add components to solution. In the present
3.1.4 continuous flow, n—the continual replacement of
context, dissolution of the glass may proceed at a steady-state
solution in the reaction cell with fresh test solution.
ratethatisfixedbythesolutionflowrate,temperature,solution
3.1.5 control test, n—test conducted without specimen to
pH, and other rate-affecting processes.
measure background concentrations in the test solution and
3.1.23 stoichiometric dissolution, n—release of elements
from interactions between test solution and apparatus.
into solution in the same proportion that they are in the glass.
3.1.6 crushed glass, n—small particles of glass produced by
3.1.24 test solution, n—the solution entering the reaction
mechanically fracturing larger pieces of glass.
cell.
3.1.7 dissolution, n—the result of reactions in which chemi-
cal bonds are broken and species are released from the glass
4. Summary of Practice
and become dissolved in the test solution.
4.1 Crushed or monolithic glass specimens having a known
3.1.8 effluent solution, n—the solution exiting the reaction
surfaceareaarecontactedbyasolutionthatcontinuouslyflows
cell.
at a known flow rate and at a constant temperature through a
3.1.9 fines, n—small pieces of glass that adhere to the glass
reaction cell that contains the glass sample. The concentration
particles prepared for use in the test that are not removed by
of a soluble glass component (i) in the effluent solution exiting
sieving.
the sample cell is used to calculate the amount of glass that has
3.1.10 forward glass dissolution rate, n—the rate at which
dissolved. The flow rate is determined by dividing the mass of
glass dissolves into solution at specific values of the tempera-
solution that is collected for analysis by the duration over
ture and pH in the absence of back reactions.
which it was collected. The dissolution rate of the glass is
3.1.11 gravimetric, adj—measured by change in mass.
calculated by using Eq 1:
3.1.12 high-purity water, n—ASTMType I orType II water
F
withamaximumtotalmattercontentincludingsolublesilicaof
@C ~i!– C°#·
S D
i i
S°
0.1 g/m and a minimal electrical resistivity of 16.67 MV•cm
rate 5 (1)
f
i
at 25°C (see Specification D1193 and Terminology D1129).
where Ci (i) is the steady-state concentration of component
3.1.13 influent solution, n—the solution entering the reac-
i measured in the effluent solution, Ci° is the background
tion cell.
concentration of component i in the influent solution measured
3.1.14 intrinsic rate constant, n—the component of the
in a blank test, F is the solution flow rate, S° is the initial
forward rate constant that depends only on the glass composi-
surface area of the glass sample that is exposed to solution, and
tion
fi is the mass fraction of component i in the glass. Several
3.1.15 leached layer, n—residual material at the glass
samples of the effluent solution are collected during the test to
surface from which some or all soluble components have been
determine the steady-state concentrations of dissolved glass
leached.
componentsataparticularsolutionflowrate.Becausetheglass
3.1.16 leaching , n—the preferential loss of soluble compo-
dissolution rate will likely be affected by the steady-state
nents from a material.
concentrations of dissolved silica and other solutes, tests must
3.1.17 mesh size fraction , n—a designation of the size
be conducted at several solution flow rates to provide data that
rangeofcrushedglassgivenbythecombinationofthesmallest
can be extrapolated to zero concentration to determine the
mesh size that the glass is passed through (prefixed by a
forward glass dissolution rate at infinite dilutions.
negative sign) and the largest mesh size that it does not pass
through(prefixedbyapositivesign).Forexample,the–40+60
5. Significance and Use
mesh size fraction will pass through a 40 mesh sieve but will
not pass through a 60 mesh sieve. 5.1 This practice provides a prescriptive description of the
3.1.18 pulsed flow, n—the replacement of solution in the design of a SPFT test apparatus and identifies aspects of the
reaction cell with fresh test solution due to the regular periodic performanceofSPFTtestsandinterpretationoftestresultsthat
action of a mechanical pump. Excludes manual replacement of must be addressed by the experimenter to provide confidence
the test solution. in the measured dissolution rate.
3.1.19 reaction cell, n—the container in which the sample 5.2 The SPFT test method described in this practice can be
remains during the test. usedtocharacterizevariousaspectsofglasscorrosionbehavior
C1662–07
that can be utilized in a mechanistic model for calculating leached layers and secondary phases were formed on the
long-term behavior of a nuclear waste glass. specimen surface, and so forth. These occurrences may impact
5.3 Depending on the values of test parameters that are theaccuracyoftheglassdissolutionratethatismeasuredusing
used, the results of SPFT tests can be used to measure the thismethod.Thispracticedoesnotaddresstheanalysisofsolid
intrinsic dissolution rate of a glass, the temperature and pH reaction materials.
dependencies of the rate, and the effects of various dissolved
6. Procedure
species on the dissolution rate.
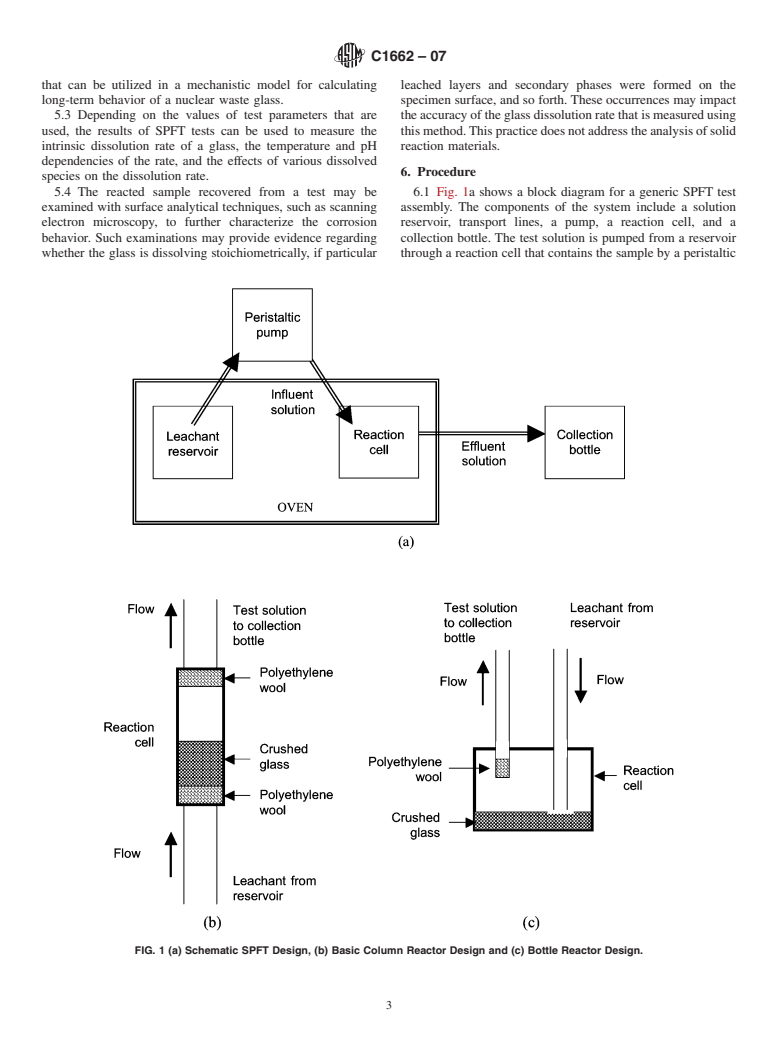
5.4 The reacted sample recovered from a test may be 6.1 Fig. 1a shows a block diagram for a generic SPFT test
examined with surface analytical techniques, such as scanning assembly. The components of the system include a solution
electron microscopy, to further characterize the corrosion reservoir, transport lines, a pump, a reaction cell, and a
behavior. Such examinations may provide evidence regarding collection bottle. The test solution is pumped from a reservoir
whether the glass is dissolving stoichiometrically, if particular through a reaction cell that contains the sample by a peristaltic
FIG. 1 (a) Schematic SPFT Design, (b) Basic Column Reactor Design and (c) Bottle Reactor Design.
C1662–07
pump or similar device. Depending on the temperature of monoliths to be used in the series of tests shall be consistent
interest, the reaction cell may be located in a constant tem- and shall be reported with the test results. For example, if the
perature oven or water bath. The leachant in the reservoir can
faces of the samples are polished with silica carbide paper, the
be heated to the test temperature in the same oven.As influent
grit and lubricating fluid shall be reported. To facilitate
solution is pumped into the reaction cell, an equal volume of
comparison of tests with different glasses, a final polish of
effluent solution will be displaced from the reaction cell. The
600-grit is recommended.
effluent solution is sampled several times during the test for
6.5 Themassfractionsofelementalsiliconintheglassmust
analysis. The mass of effluent that is collected for analysis and
be known to determine the glass dissolution rate (see also
the collection time are used to calculate the solution flow rate
9.4.5). This may be determined by direct analysis of the glass
for that aliquot. Chemical analysis of the effluent solution is
(see Test Method C169) or based on the as-batched composi-
performed to measure the concentration of the components
tion of the glass.
used to calculate the dissolution rate. The concentrations of
6.6 The flow rate of the solution through the reaction cell is
several glass components can be tracked to determine whether
calculatedbydividingthemassoftestsolutioncollectedbythe
the glass is dissolving stoichiometrically. Separate tests are
duration over which it was collected.Although the flow rate is
conductedatseveralflowratesandwithseveralsamplesurface
setbeforethesampleisplacedinthereactioncell,theflowrate
areas to measure the effect of the solution composition (pri-
measured with the sample in place is used for the calculations.
marily the dissolved silica concentration) on the measured
The flow rate is likely to vary slightly with each aliquot that is
glass dissolution rate.
taken during a test. A test is acceptable if the flow rates
6.2 Either column-type or bottle-type reaction cells can be
determinedforthealiquotscollectedduringatestvaryby10 %
used; these are shown schematically in Fig. 1. In the column
or less. Samplings in which the flow rate differs by more than
cell design, the influent solution is pumped (usually upwards)
10 % shall not be used in determining the steady-state concen-
through the crushed glass (or around a monolithic sample). In
trations for that test. The average flow rate measured in a test
the bottle design, the influent solution is pumped into a cell
is used as the flow rate to calculate the glass dissolution rate in
filled with solution and displaces an equal volume of effluent
that test.
solution. Polyethylene wool or an equivalent material can be
6.7 Asmall change in the solution concentration may occur
usedtopreventcrushedglassparticlesfrombeingflushedfrom
the reaction cell during the test, or the effluent s
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.