ASTM D7318-07
(Test Method)Standard Test Method for Total Inorganic Sulfate in Ethanol by Potentiometric Titration
Standard Test Method for Total Inorganic Sulfate in Ethanol by Potentiometric Titration
SIGNIFICANCE AND USE
Ethanol is used as a blending agent added to gasoline. Sulfates are indicated in filter plugging deposits and fuel injector deposits. When fuel ethanol is burned, sulfates may contribute to sulfuric acid emissions. Ethanol acceptability for use depends on the sulfate content. Sulfate content, as measured by this test method, can be used as one measure of determination of the acceptability of ethanol for automotive spark-ignition engine fuel use.
SCOPE
1.1 This test method covers a potentiometric titration procedure for determining the total inorganic sulfate content of hydrous, anhydrous ethanol, and anhydrous denatured ethanol, which is added as a blending agent with spark ignition fuels. It is intended for the analysis of denatured ethanol samples containing between 1.0-20 mg/kg total inorganic sulfate.
1.2 The values stated in SI units are to be regarded as the standard. The values given in parentheses are for information only.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Material Safety Data Sheets are available for reagents and materials. Review them for hazards prior to usage.
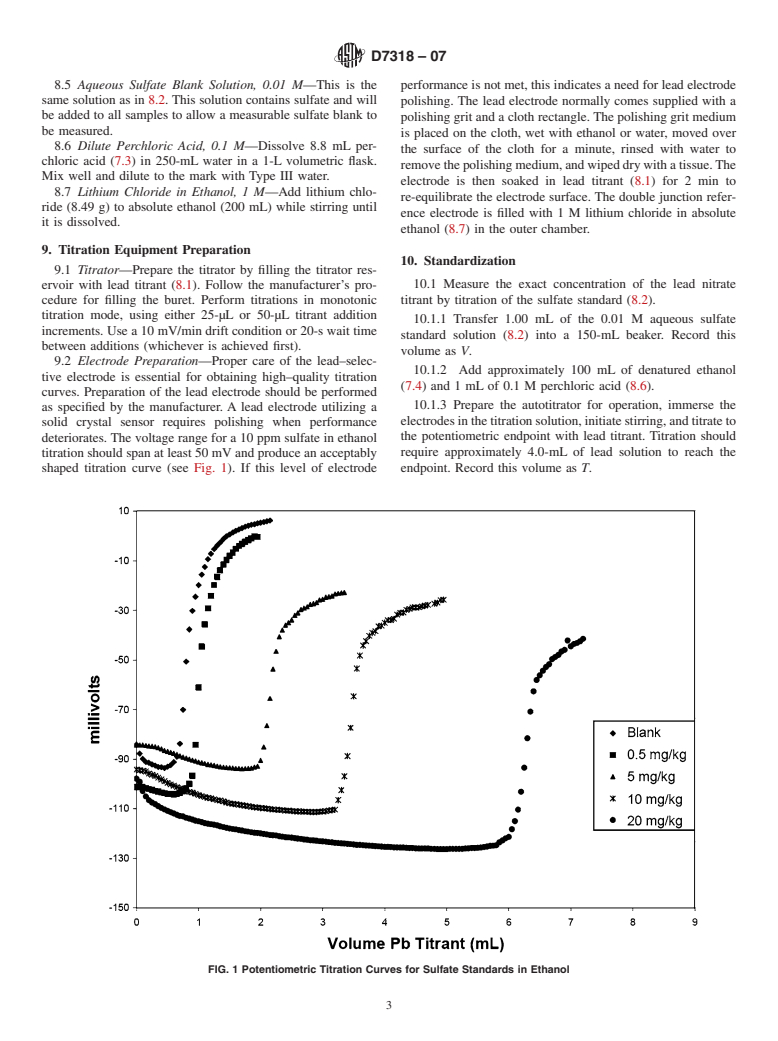
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D7318–07
Standard Test Method for
Total Inorganic Sulfate in Ethanol by Potentiometric
Titration
This standard is issued under the fixed designation D7318; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3. Terminology
1.1 This test method covers a potentiometric titration pro- 3.1 Definitions:
cedure for determining the total inorganic sulfate content of 3.1.1 inorganic sulfate, n—sulfate species present as sulfu-
hydrous, anhydrous ethanol, and anhydrous denatured ethanol, ric acid, ionic salts of this acid, or mixtures of these.
which is added as a blending agent with spark ignition fuels. It 3.1.1.1 Discussion—Specifically in this test method, inor-
is intended for the analysis of denatured ethanol samples ganic sulfate is present as sulfate in ethanol.
containing between 1.0–20 mg/kg total inorganic sulfate.
4. Summary of Test Method
1.2 The values stated in SI units are to be regarded as the
4.1 An ethanol sample containing inorganic sulfate is
standard. The values given in parentheses are for information
only. titrated in ethanolic medium with a standard lead nitrate
solution. Lead sulfate precipitate is formed during the titration.
1.3 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the Perchloric acid is added to remove possible interference from
carbonate. The endpoint is signaled by an increase in lead ion
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica- activity, as measured by a lead–selective electrode.
bility of regulatory limitations prior to use. Material Safety
5. Significance and Use
Data Sheets are available for reagents and materials. Review
5.1 Ethanol is used as a blending agent added to gasoline.
them for hazards prior to usage.
Sulfates are indicated in filter plugging deposits and fuel
2. Referenced Documents
injector deposits. When fuel ethanol is burned, sulfates may
2.1 ASTM Standards: contribute to sulfuric acid emissions. Ethanol acceptability for
use depends on the sulfate content. Sulfate content, as mea-
D1193 Specification for Reagent Water
D4052 Test Method for Density, Relative Density, and API sured by this test method, can be used as one measure of
determination of the acceptability of ethanol for automotive
Gravity of Liquids by Digital Density Meter
D4057 Practice for Manual Sampling of Petroleum and spark-ignition engine fuel use.
Petroleum Products
6. Apparatus
D4177 Practice for Automatic Sampling of Petroleum and
6.1 Potentiometric Titration Assembly—A titration assem-
Petroleum Products
bly consisting of an automatic titrator fitted with a lead
D6299 Practice for Applying Statistical Quality Assurance
ion-selective electrode, a double-junction reference electrode,
and Control Charting Techniques to Evaluate Analytical
buret, and stirring is used. Stirring may be accomplished by
Measurement System Performance
means of magnetic or propeller type stirrer mechanisms. The
buret size should ideally be 10 or 20 mL.
This test method is under the jurisdiction of ASTM Committee D02 on
6.2 Reference Electrode—Adouble junction reference elec-
Petroleum Products and Lubricants and is the direct responsibility of Subcommittee
trode with the inner electrode composed of silver/silver chlo-
D02.03 on Elemental Analysis.
ride with a potassium chloride solution as internal electrolyte.
Current edition approved Feb. 1, 2007. Published March 2007. DOI: 10.1520/
D7318-07.
The external solution is composed of 1 M lithium chloride in
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
ethanol. This configuration is used to prevent silver ion, a lead
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
electrodepoison,fromleachingintotheanalytesolutionduring
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. titration. Preferred electrolytes for use in double junction
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D7318–07
electrodes may vary with the manufacturer; use the manufac- Accurately weigh about 0.70 g on an analytical balance to the
turer’s recommended electrolytes for the application. Other nearest tenth of a milligram, and place it in a 500-mL
types of reference electrodes may be considered with some
volumetric flask. Add Type III water to dissolve the sodium
caveats (for example, single junction, combination, or glassy
sulfate, then dilute to volume. Calculate the exact concentra-
carbon), but the data presented in this test method were
tion in accordance with Eq 1.
generated using exclusively a double junction electrode, which
G
is the best choice for this determination.
5 Molarity (1)
~142.02!~0.500!
6.3 Lead Electrode—Aleadsulfide–basedcrystallinesensor
type lead ion selective electrode (ISE) is used.
6.4 Drying Oven—Adrying oven for drying sodium sulfate Molarity = molarity of sulfate standard solution, mol/L,
at 110°C is required. G = weight in grams of Na SO , dissolved in 500
2 4
6.5 Pipets or Volumetric Transferring Devices—Class A mL, and
142.02 = gram molecular weight of Na SO .
glass pipets or their equivalent.
2 4
6.6 Polishing Material—Lead sulfide based crystalline sen-
8.3 Aqueous Sulfate Stock Solution for Standards in Etha-
sor electrodes require polishing to remove oxidation products.
nol, 2000 mg/L—Accurately weigh 2.95 g anhydrous sodium
These materials are supplied with the electrode from the
sulfate to the nearest tenth of a milligram and transfer it to a
manufacturer.
1-L volumetric flask. (Dried anhydrous sodium sulfate should
6.7 pH Test Strips—Test strips in the range of pH 1 to pH 7.
be stored in a desiccator.) Add Type III water to dissolve the
6.8 Titration Vessels—Standard glass beakers or titration
sodium sulfate, and make to volume. Calculate the concentra-
vessels supplied with titration equipment.
tion of sulfate in the solution in accordance with Eq 2.
7. Reagents and Materials
gNa SO 0.6764 1000 mg/g
~ ! ~ ! ~ !
2 4
Aqueous Stock Sulfate ~mg/L!5
1L
7.1 Lead Nitrate—Reagent grade, 99% minimum purity.
(2)
(Warning—Poison, harmful by inhalation and if ingested.
Avoid contact with the skin.) Dispose of this material in
accordance with accepted local requirements.
gNa SO = weight in grams of Na SO dissolved in 1 L,
2 4 2 4
7.2 Sodium Sulfate—Anhydrous, reagent grade, 99% mini-
and
mum purity. (Warning—Do not ingest. Avoid unnecessary
0.6764 = fraction of sulfate in Na SO .
2 4
exposure.)
8.4 Sulfate Standards in Ethanol—Ethanol (denatured con-
7.3 Perchloric Acid 70%—A.C.S. reagent grade, minimum
taining no measurable sulfate) is weighed into a container
purity with sulfate concentration <0.001% (m/M). Dispose of
(equipped with a closure to prevent evaporation) in accordance
thismaterialinaccordancewithacceptedlocalrequirements.It
with Table 1 to achieve the desired standard. Aqueous sulfate
must contain no measurable sulfate. (Warning—Corrosive;
stock solution from 8.3 is added to the solution in accordance
keep away from skin and eyes. Perchloric acid is a strong
with Table 1, and the final weight of the solution recorded.
oxidizer.)
Standards should be remade weekly or if recovery of less than
7.4 Ethanol—Denatured with methanol, formula 3A or
90% is noted. The concentration of the standard is calculated
histological grade ethanol, anhydrous, denatured with ethyl
by dividing the number of milligrams sulfate from the sulfate
acetate, methyl isobutyl ketone and hydrocarbon naphtha. It
stock solution and dividing by the final solution weight in
must be free of any measurable sulfate. (Warning—
Flammable, toxic, may be harmful or fatal if ingested or accordance with Eq 3.
inhaled. Avoid skin contact.)
V 3 C
EtOH Sulfate Standard ~mg/kg!5 (3)
7.5 Ethanol—Absolute, 200 proof, 99.5%, A.C.S. reagent
W
grade.
7.6 Lithium Chloride—99+%, A.C.S. reagent grade.
V = volume of aqueous sulfate stock (8.3), mL,
7.7 Water—Type III reagent water conforming to Specifi-
C = concentration of aqueous sulfate stock (8.3), mg/L,
cation D1193.
and
7.8 Anhydrous Calcium Sulfate Desiccant.
W = final weight of ethanol and aqueous sulfate stock
8. Preparation of Standard Solutions aliquot, g.
8.1 Lead Nitrate Titrant, 0.0025 M—Dissolve 0.833 g lead
nitrate in 300 mL water. Pour into a 1-L bottle and fill with
denatured ethanol and mix well. Standardize in accordance
TABLE 1 Preparation of Sulfate Standards in Ethanol
with 10.1.
Ethanolic Sulfate Standard, Ethanol, Aqueous Sulfate Stock
8.1.1 Alternatively, this solution may be purchased from a
mg sulfate/kg ethanol g Solution, mL
commercial vendor, and its exact molarity shall be determined
50 975 25
in accordance with 10.1. 20 990 10
10 995 5
8.2 Aqueous Sulfate Standard, 0.01 M—Dry 5 g anhydrous
5 997.5 2.5
sodium sulfate at 110°C for 1 h. Remove it from the oven, and
1 999.5 0.5
allow it to cool in a desiccator over anhydrous calcium sulfate.
D7318–07
8.5 Aqueous Sulfate Blank Solution, 0.01 M—This is the performance is not met, this indicates a need for lead electrode
same solution as in 8.2. This solution contains sulfate and will polishing. The lead electrode normally comes supplied with a
be added to all samples to allow a measurable sulfate blank to
polishing grit and a cloth rectangle. The polishing grit medium
be measured.
is placed on the cloth, wet with ethanol or water, moved over
8.6 Dilute Perchloric Acid, 0.1 M—Dissolve 8.8 mL per-
the surface of the cloth for a minute, rinsed with water to
chloric acid (7.3) in 250-mL water in a 1-L volumetric flask.
removethepolishingmedium,andwipeddrywithatissue.The
Mix well and dilute to the mark with Type III water.
electrode is then soaked in lead titrant (8.1) for 2 min to
8.7 Lithium Chloride in Ethanol, 1 M—Add lithium chlo-
re-equilibrate the electrode surface. The double junction refer-
ride (8.49 g) to absolute ethanol (200 mL) while stirring until
ence electrode is filled with 1 M lithium chloride in absolute
it is dissolved.
ethanol (8.7) in the outer chamber.
9. Titration Equipment Preparation
10. Standardization
9.1 Titrator—Prepare the titrator by filling the titrator res-
ervoir with lead titrant (8.1). Follow the manufacturer’s pro- 10.1 Measure the exact concentration of the lead nitrate
cedure for filling the buret. Perform titrations in monotonic titrant by titration of the sulfate standard (8.2).
titration mode, using either 25-µL or 50-µL titrant addition
10.1.1 Transfer 1.00 mL of the 0.01 M aqueous sulfate
increments. Use a 10 mV/min drift condition or 20-s wait time
standar
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.