ASTM F2091-01
(Specification)Standard Specification for Acetabular Prostheses
Standard Specification for Acetabular Prostheses
SCOPE
1.1 This standard describes acetabular resurfacing devices used to provide a functioning articulation between the bones of the acetabulum and the femur.
1.2 This standard is intended to provide basic descriptions of materials and device geometry. Additionally, those characteristics determined to be important to in vivo performance of the device are defined.
1.3 Acetabular prostheses included within the scope of this standard are intended for mechanical fixation between the prosthesis and host bone, by the use of bone cement or through biological fixation.
1.4 Custom (designed explicitly for a single patient), revision, or constrained acetabular prostheses are not covered within the scope of this standard.
1.5 This standard does not cover the details for quality assurance, design control, production control contained in 21 CFR 820 (Quality System Regulation) and ISO 9001.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F 2091 – 01
Standard Specification for
Acetabular Prostheses
This standard is issued under the fixed designation F 2091; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope F 562 Specification for Wrought Cobalt-35 Nickel-20
Chromium-10 Molybdenum Alloy for Surgical Implant
1.1 This standard describes acetabular resurfacing devices
Applications (UNS R30035)
used to provide a functioning articulation between the bones of
F 563 Specification for Wrought Cobalt-Nickel-Chromium-
the acetabulum and the femur.
Molybdenum Tungsten-Iron Alloy for Surgical Implant
1.2 This standard is intended to provide basic descriptions
Applications
of materials and device geometry. Additionally, those charac-
F 601 Practice for Fluorescent Penetrant Inspection of Me-
teristics determined to be important to in vivo performance of
tallic Surgical Implants
the device are defined.
F 603 Specification for High-Purity Dense Aluminum Ox-
1.3 Acetabular prostheses included within the scope of this
ide for Surgical Implant Application
standard are intended for mechanical fixation between the
F 629 Practice for Radiography of Cast Metallic Surgical
prosthesis and host bone, by the use of bone cement or through
Implants
biological fixation.
F 648 Specification for Ultra-High-Molecular-Weight Poly-
1.4 Custom (designed explicitly for a single patient), revi-
ethylene Powder and Fabricated Form for Surgical Im-
sion, or constrained acetabular prostheses are not covered
plants
within the scope of this standard.
F 745 Specification for 18 Chromium-12.5 Nickel-2.5 Mo-
1.5 This standard does not cover the details for quality
lybdenum Stainless Steel for Cast and Solution-Annealed
assurance, design control, production control contained in 21
Surgical Implant Applications
CFR 820 (Quality System Regulation) and ISO 9001.
F 746 Test Method for Pitting or Crevice Corrosion of
2. Referenced Documents Metallic Surgical Implant Materials
F 748 Practice for Selecting Generic Biological Test Meth-
2.1 ASTM Standards:
ods for Materials and Devices
F 67 Specification for Unalloyed Titanium for Surgical
F 799 Specification for Cobalt-28 Chromium-6 Molybde-
Implant Applications
num Alloy Forgings for Surgical Implants (UNS R31537,
F 75 Specification for Cobalt-28 Chromium-6 Molybdenum
R31538, R31539)
Casting Alloy and Cast Products for Surgical Implants
F 981 Practice for Assessment of Compatibility of Bioma-
(UNS R30075)
terials for Surgical Implants with Respect to Effect of
F 86 Practice for Surface Preparation and Marking of Me-
Materials on Muscle and Bone
tallic Surgical Implants
F 983 Practice for Permanent Marking of Orthopedic Im-
F 90 Specification for Wrought Cobalt-20 Chromium-15
plant Components
Tungsten-10 Nickel Alloy for Surgical Implant Applica-
F 1044 Test Method for Shear Testing of Calcium Phos-
tions (R30605)
phate Coatings and Metallic Coatings
F 136 Specification for Wrought Titanium-6 Aluminum-4
F 1108 Specification forTitanium-6Aluminum-4Vanadium
Vanadium ELI (Extra Low Interstitial) Alloy (UNS
Alloy Castings for Surgical Implants (UNS R56406)
R56401) for Surgical Implant Applications
F 1147 Test Method for Tension Testing of Porous Metal
F 138 Specification for Wrought 18 Chromium-14 Nickel-
Coatings
2.5 Molybdenum Stainless Steel Bar and Wire for Surgical
F 1160 Test Method for Sheer and Bending Fatigue Testing
Implants (UNS S31673)
of Calcium Phosphate and Metallic Medical Coatings
F 1185 Specification for Composition of Ceramic Hydroxy-
1 2
This specification is under the jurisdiction of ASTM Committee F04 on
lapatite for Surgical Implants
Medical and Surgical Materials and Devices and is the direct responsibility of
F 1377 Cobalt-28 Chromium-6 Molybdenum Powder for
Subcommittee F04.22 on Arthroplasty.
Coating of Orthopedic Implants (UNS R30075)
Current edition approved June 10, 2001. Published September 2001.
Annual Book of ASTM Standards, Vol 13.01.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
F2091–01
F 1472 Specification for Wrought Titanium-6 Aluminum-4 pore size between 100 to 1000 µm, and a thickness between
Vanadium Alloy for Surgical Implant Applications (UNS 500 to 1500 µm. This porous layer may be manufactured
R56400) directly into the device by casting or by various electro/
F 1501 Test Method for Tension Testing of Calcium Phos- chemical/thermal/mechanical means, or applied as a coating of
phate Coatings particles, beads, or mesh by processes such as sintering or
F 1537 Specification for Wrought Cobalt-28 Chromium-8 plasma spray.
Molybdenum Alloy for Surgical Implants (UNS R31537, 3.1.6 radiographic marker, n—nonstructural, generally thin
UNS R31538, and UNS R31529) wire, designed to be apparent on X-rays taken after placement
F 1580 Specification for Titanium and Titanium-6 % of implants that otherwise would be unapparent on such
Aluminum-4 % Vanadium Alloy Powders for Coatings of X-rays.
Surgical Implants 3.1.7 retention element, n—any ring, taper, wire, or other
F 1714 Guide for Gravimetric Wear Assessment of Pros- protrusion or cavity from the interior surface of the shell or the
thetic Hip Designs in Simulator Devices exterior surface of the bearing element that is intended to affix
F 1820 TestMethodforDeterminingtheAxialDisassembly the bearing element to the shell.
Force of a Modular Acetabular Device 3.1.8 shell, n—metal structure supporting the articulating
F 1978 Test Method for Measuring Abrasion Resistance of surface material, and which may be fixed rigidly to the
Metallic Thermal Spray Coatings by Using the Tabery articulating surface or fixed such that it allows the articulating
Abraser surface to rotate or translate.
F 2033 Specification for Total Hip Joint Prosthesis and Hip 3.1.9 surface texturing, n—repetitive or random deviations
Endoprosthesis Bearing Surfaces Made of Metallic, Ce- from the nominal surface that forms the three dimensional
ramic, and Polymeric Materials topography of the surface.
2.2 ISO Standards: 3.2 Dimensions of acetabular prostheses should be desig-
ISO 5832 Implants for surgery—Metallic materials for sur- nated in accordance with Figs. 1-3 or by an equally acceptable
gical implants and detailed method.
ISO 5834 Implants for surgery—Ultra high molecular
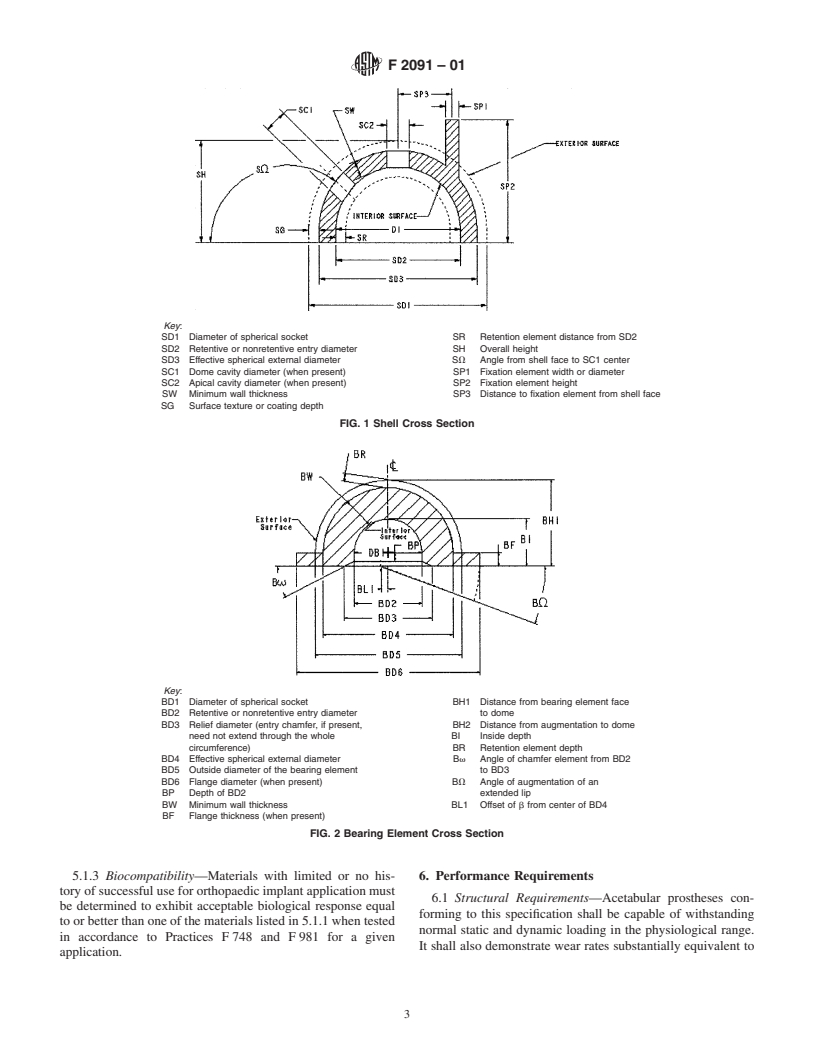
NOTE 1—Figs. 1-3 are intended to be illustrative of typical acetabular
weight polyethylene
prostheses and to designate dimensions, but representation of the compo-
ISO 6474 Implants for surgery—Ceramic materials based
nents does not otherwise form part of the standard.
on alumina
4. Types
ISO 9001 Quality systems—Model for quality assurance in
design/development, production, installation, and servic-
4.1 Acetabular prostheses falling within the scope of this
ing
specification are of two types, as defined below. There are no
2.3 Code of Federal Regulations:
distinguishing features (for example, augmentation or lack
21 CFR 820 Quality System Regulation
thereof,holes,andsoforth)thatwouldexemptanydevicefrom
any requirement of this specification.
3. Terminology
4.1.1 Type I—Single-piece acetabular prostheses.
3.1 Definitions:
NOTE 2—Specifications to both bearing elements and shell may apply.
3.1.1 bearing element, n—articulating surface element be-
4.1.2 Type II—Multipiece, modular structure prostheses.
tween the femoral head and shell or bonding agent (bone
cement).
5. Material
3.1.2 cavity, n—any slot, cut, hole, or other feature within
5.1 The choice of materials is understood to be a necessary,
the shell intended to accommodate modular adjunct fixation
but not sufficient, assurance of function of the device made
elements; instruments for insertion, extraction, and so forth; or
fromthem.Alldevicesconformingtothisspecificationshallbe
for manufacturing purposes.
fabricated from materials with adequate mechanical strength
3.1.3 fixation element, n—any peg, spike, threadform, or
and durability, corrosion resistance, and biocompatibility.
other protrusion from the exterior surface of the shell intended
5.1.1 Mechanical Strength—Various components of ac-
toincreasethesurfacecontactormechanicalinterlockbetween
etabular prostheses have been successfully fabricated from the
thecomponent,thebondingagent,orthenaturalacetabulumor
followingmaterials:SeeSpecificationsF 67,F 75,F 90,F 136,
a combination thereof.
F 138, F 562, F 563, F 603, F 648, F 745, F 799, F 1108,
3.1.4 flange, n—rim extending from the entry diameter of
F 1185, F 1377, F 1472, F 1537, F 1580; and ISO 5832, ISO
bearing element.
5834, and ISO 6474. However, not all of these materials may
3.1.5 porous coating, n—a region on the exterior surface of
possess sufficient mechanical strength for critical highly
the shell characterized by interconnecting subsurface pores,
stressed components nor for articulating surfaces. Associated
generally with volume porosity between 30 to 70 %, average
standards include Practices F 601 and F 629.
5.1.2 Corrosion Resistance—Materials with limited or no
history of successful use for orthopaedic implant application
Available from International Organization for Standardization, 1 Rue de
must be determined to exhibit corrosion resistance equal to or
Varembé, Case Postale 56, CH-1211, Geneva 20, Switzerland.
better than one of the materials listed in 5.1.1 when tested in
AvailablefromStandardizationDocumentsOrderDesk,Bldg.4SectionD,700
Robbins Ave, Philadelphia, PA 19111-5094, Attn: NPODS. accordance to Test Method F 746.
F2091–01
Key:
SD1 Diameter of spherical socket SR Retention element distance from SD2
SD2 Retentive or nonretentive entry diameter SH Overall height
SD3 Effective spherical external
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.