ASTM D4783-01(2021)
(Test Method)Standard Test Methods for Resistance of Adhesive Preparations in Container to Attack by Bacteria, Yeast, and Fungi
Standard Test Methods for Resistance of Adhesive Preparations in Container to Attack by Bacteria, Yeast, and Fungi
SIGNIFICANCE AND USE
5.1 These test methods are used to demonstrate whether an adhesive preparation is sufficiently protected with biocide to resist attack by bacteria, yeast, and fungi during its storage life. They are patterned after methods used by biological laboratories serving the adhesive industry.
5.2 These test methods may also be used to determine the efficacy of different biocide systems against specific microorganisms.
5.3 These test methods are especially useful when tested against wild-type microorganisms which have been isolated from contaminated adhesives as an aid in determining the amount and type of biocide necessary to kill or inhibit the growth of the contaminants. If an isolated microorganism not generally used as a challenge organism, is chosen as the inoculum, it is important to identify the organism and determine on which medium and under what conditions it will grow, in order to demonstrate the efficacy of the biocide.
5.4 The results obtained when using the procedures given in these methods apply only to the species which are used for the testing. The test species listed in Section 9 are frequently used by laboratories to test for antimicrobial properties, but they are not the only ones which could be used. Selection of the species to use for these test methods requires informed judgment by the testing laboratory or by the party requesting the tests. It is also important that species which commonly attack adhesives be used. See 9.4.
5.5 The presence of an active biocide carried over from the adhesive specimen to the agar could have an inhibiting effect on the growth of microorganisms, resulting in no growth during the span of a normal incubation period, when in fact, viable microorganisms are present, but their growth has been slowed down or held in stasis. The use of Letheen agar and broth is recommended to neutralize the effect of this carry-over.
Note 4: Letheen agar may be used for the streak plates, or if another agar is chosen for testing, ...
SCOPE
1.1 These test methods cover the determination of the resistance of liquid adhesive preparations to microbial attack in the container by challenging adhesive specimens with cultures of bacteria, yeast, or fungi, and checking for their ability to return to sterility. These test methods return qualitative results.
1.2 The values stated in SI units are to be regarded as the standard. The values in parentheses are for information only.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use. These test methods are designed to be used by persons trained in correct microbiological technique. Specific precautionary statements are given in Section 8.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: D4783 − 01 (Reapproved 2021)
Standard Test Methods for
Resistance of Adhesive Preparations in Container to Attack
by Bacteria, Yeast, and Fungi
This standard is issued under the fixed designation D4783; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
1. Scope D4300Test Methods for Ability of Adhesive Films to
Support or Resist the Growth of Fungi
1.1 These test methods cover the determination of the
E640Test Method for Preservatives in Water-Containing
resistanceofliquidadhesivepreparationstomicrobialattackin
Cosmetics
the container by challenging adhesive specimens with cultures
of bacteria, yeast, or fungi, and checking for their ability to
NOTE 1—Test Method E640 is under the jurisdiction of ASTM
Committee E35 on Pesticides. The procedure in this method outlines a
return to sterility.These test methods return qualitative results.
serial dilution method of determining plate count using a pour plate
1.2 The values stated in SI units are to be regarded as the
technique.
standard. The values in parentheses are for information only.
2.2 TAPPI Method:
1.3 This standard does not purport to address all of the
T487Fungus Resistance of Paper and Paperboard
safety concerns, if any, associated with its use. It is the
2.3 CSMA:
responsibility of the user of this standard to establish appro-
Cosmetics Preservation, Method38
priate safety, health, and environmental practices and deter-
mine the applicability of regulatory limitations prior to use.
3. Terminology
These test methods are designed to be used by persons trained
3.1 Definitions—Many terms in these test methods are
in correct microbiological technique. Specific precautionary
defined in Terminology D907.
statements are given in Section 8.
3.2 Definitions of Terms Specific to This Standard:
1.4 This international standard was developed in accor-
3.2.1 adhesive preparation, n—theadhesiveaspackagedfor
dance with internationally recognized principles on standard-
distribution, storage, and use.
ization established in the Decision on Principles for the
3.3 Abbreviations:
Development of International Standards, Guides and Recom-
mendations issued by the World Trade Organization Technical 3.3.1 PBS—phosphate buffered saline.
Barriers to Trade (TBT) Committee.
3.3.2 PDA—potato dextrose agar.
3.3.3 YMPG—yeast malt peptone glucose (agar).
2. Referenced Documents
2.1 ASTM Standards:
4. Summary of Test Method
D907Terminology of Adhesives
4.1 The adhesive specimen is challenged by inoculation
D4299TestMethodforEffectofBacterialContaminationon
with a culture of bacteria, yeast, or fungi, which may be a
Performance of Adhesive Preparations and Adhesives
single species or a mixed culture of several species, following
Films (Withdrawn 1990)
the guidelines given in Note 6. The inoculated adhesive
specimenisstoredat21to27°C(70to80°F)for7days,during
whichtimecultures(streakplates)aremadeatpresetintervals.
These test methods are under the jurisdiction of ASTM Committee D14 on
See Note 2. At any point in the series of challenges, if the
Adhesives and are the direct responsibility of Subcommittee D14.30 on Wood
Adhesives. inoculated specimen shows microbial growth on the streak
Current edition approved April 1, 2021. Published April 2021. Originally
plates made during the week following the challenge (indicat-
approved in 1988. Last previous edition approved in 2013 as D4783–01 (2013).
ing that it has not returned to sterility), the test is discontinued,
DOI: 10.1520/D4783-01R21.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on Available from Technological Association of the Pulp and Paper Industry
the ASTM website. (TAPPI), 15 Technology Parkway South, Suite 115, Peachtree Corners, GA30092,
The last approved version of this historical standard is referenced on http://www.tappi.org.
www.astm.org. This method is the same as Test Method E640.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D4783 − 01 (2021)
checks are shown in Table 1 and Table 2, Schedule A for bacteria and
and the sample is reported as not resistant to attack in the
yeast,andScheduleBforfungi.Theexactformattobefollowedwillvary,
container by the species or combination of species used as the
according to the convenience of the schedule to the testing laboratory and
inoculum. If the cultures show no growth, the test is repeated
special circumstances relating to the problem being addressed.
with up to four challenges. If the specimen tests out as sterile
NOTE 3—A serial-dilution plate-count method of checking for sterility
following the fourth challenge, it is reported to be resistant to
may be used when numerical information is needed on the population of
viable organisms or the reduction in population with increasing levels of
attackinthecontainerbythespeciesorcombinationofspecies
biocide. Letheen broth is recommended for the diluent and Letheen agar
of bacteria, fungi, or yeast used as the inoculum. At the
for the pour plate. See Note 1.
discretion of the biological laboratory, the test may be discon-
tinued after the second or third challenge. See Section 16 for
5. Significance and Use
further interpretation.
5.1 These test methods are used to demonstrate whether an
4.2 The time necessary to kill is determined by noting the
adhesive preparation is sufficiently protected with biocide to
earliest streak plate to read sterile. If the 4-h plate is positive
resistattackbybacteria,yeast,andfungiduringitsstoragelife.
and the 24-h plate is negative, the kill time could be narrowed
They are patterned after methods used by biological laborato-
down further by repeating the challenge and making streak
ries serving the adhesive industry.
platesatintervalsof4,8,12,and24hfollowingthechallenge.
5.2 These test methods may also be used to determine the
4.3 The testing laboratory has the option of changing the
efficacy of different biocide systems against specific microor-
timing of the challenges, the sterility checks, and the incuba-
ganisms.
tion period.
5.3 These test methods are especially useful when tested
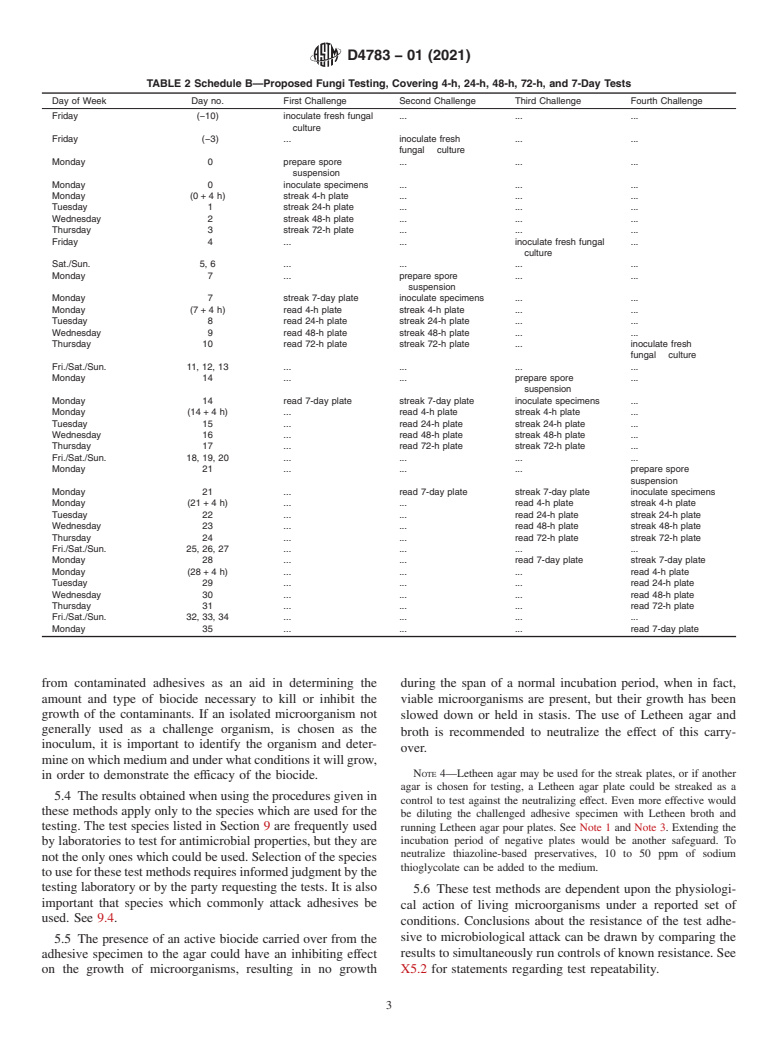
NOTE 2—Two proposed schedules for the challenging and sterility against wild-type microorganisms which have been isolated
TABLE 1 Schedule A—Proposed Bacteria and Yeast Testing, Covering 4-h, 24-h, 48-h, 72-h, and 7-Day Tests
Day of Week Day no. First Challenge Second Challenge Third Challenge Fourth Challenge
Monday (−1) inoculate fresh bacterial . . .
or yeast culture
Tuesday 0 prepare suspension . . .
Tuesday 0 inoculate specimens . . .
Tuesday (0 + 4 h) streak 4-h plate . . .
Wednesday 1 streak 24-h plate . . .
Thursday 2 streak 48-h plate . . .
Friday 3 streak 72-h plate . . .
Sat./Sun. 4–5 . . . .
Monday 6 . inoculate fresh bacterial . .
or yeast culture
Tuesday 7 . prepare suspension . .
Tuesday 7 streak 7-day plate inoculate specimens . .
Tuesday (7 + 4 h) read 4-h plate streak 4-h plate . .
Wednesday 8 read 24-h plate streak 24-h plate . .
Thursday 9 read 48-h plate streak 48-h plate . .
Friday 10 read 72-h plate streak 72-h plate . .
Sat./Sun. 11–12 . . . .
Monday 13 . . inoculate fresh bacterial .
or yeast culture
Tuesday 14 . . prepare suspension .
Tuesday 14 read 7-day plate streak 7-day plate inoculate specimens .
Tuesday (14 + 4 h) . read 4-h plate streak 4-h plate .
Wednesday 15 . read 24-h plate streak 24-h plate .
Thursday 16 . read 48-h plate streak 48-h plate .
Friday 17 . read 72-h plate streak 72-h plate .
Sat./Sun. 18–19 . . . .
Monday 20 . . . inoculate fresh
bacterial or yeast
culture
Tuesday 21 . . . prepare suspension
Tuesday 21 . read 7-day plate streak 7-day plate inoculate specimens
Tuesday (21 + 4 h) . . read 4-h plate streak 4-h plate
Wednesday 22 . . read 24-h plate streak 24-h plate
Thursday 23 . . read 48-h plate streak 48-h plate
Friday 24 . . read 72-h plate streak 72-h plate
Sat./Sun. 25–26 . . . .
Monday 27 . . . .
Tuesday 28 . . read 7-day plate streak 7-day plate
Tuesday (28 + 4 h) . . . read 4-h plate
Wednesday 29 . . . read 24-h plate
Thursday 30 . . . read 48-h plate
Friday 31 . . . read 72-h plate
Sat./Sun. 32–33 . . . .
Monday 34 . . . .
Tuesday 35 . . . read 7-day plate
D4783 − 01 (2021)
TABLE 2 Schedule B—Proposed Fungi Testing, Covering 4-h, 24-h, 48-h, 72-h, and 7-Day Tests
Day of Week Day no. First Challenge Second Challenge Third Challenge Fourth Challenge
Friday (−10) inoculate fresh fungal . . .
culture
Friday (−3) . inoculate fresh . .
fungal culture
Monday 0 prepare spore . . .
suspension
Monday 0 inoculate specimens . . .
Monday (0 + 4 h) streak 4-h plate . . .
Tuesday 1 streak 24-h plate . . .
Wednesday 2 streak 48-h plate . . .
Thursday 3 streak 72-h plate . . .
Friday 4 . . inoculate fresh fungal .
culture
Sat./Sun. 5, 6 . . . .
Monday 7 . prepare spore . .
suspension
Monday 7 streak 7-day plate inoculate specimens . .
Monday (7 + 4 h) read 4-h plate streak 4-h plate . .
Tuesday 8 read 24-h plate streak 24-h plate . .
Wednesday 9 read 48-h plate streak 48-h plate . .
Thursday 10 read 72-h plate streak 72-h plate . inoculate fresh
fungal culture
Fri./Sat./Sun. 11, 12, 13 . . . .
Monday 14 . . prepare spore .
suspension
Monday 14 read 7-day plate streak 7-day plate inoculate specimens .
Monday (14 + 4 h) . read 4-h plate streak 4-h plate .
Tuesday 15 . read 24-h plate streak 24-h plate .
Wednesday 16 . read 48-h plate streak 48-h plate .
Thursday 17 . read 72-h plate streak 72-h plate .
Fri./Sat./Sun. 18, 19, 20 . . . .
Monday 21 . . . prepare spore
suspension
Monday 21 . read 7-day plate streak 7-day plate inoculate specimens
Monday (21 + 4 h) . . read 4-h plate streak 4-h plate
Tuesday 22 . . read 24-h plate streak 24-h plate
Wednesday 23 . . read 48-h plate streak 48-h plate
Thursday 24 . . read 72-h plate streak 72-h plate
Fri./Sat./Sun. 25, 26, 27 . . . .
Monday 28 . . read 7-day plate streak 7-day plate
Monday (28 + 4 h) . . . read 4-h plate
Tuesday 29 . . . read 24-h plate
Wednesday 30 . . . read 48-h plate
Thursday 31 . . . read 72-h plate
Fri./Sat./Sun. 32, 33, 34 . . . .
Monday 35 . . . read 7-day plate
from contaminated adhesives as an aid in determining the during the span of a normal incubation period, when in fact,
amount and type of biocide necessary to kill or inhibit the
viable microorganisms are present, but their growth has been
growth of the contaminants. If an isolated microorganism not
slowed down or held in stasis. The use of Letheen agar and
generally used as a challenge organism, is chosen as the
broth is recommended to neutralize the effect of this carry-
inoculum, it is important to identify the organism and deter-
over.
mineonwhichmediumandunderwhatconditionsitwillgrow,
NOTE 4—Letheen agar may be used for the streak plates, or if another
in order to demonstrate the efficacy of the biocide.
agar is chosen for testing, a Letheen agar plate could be streaked as a
5.4 Theresultsobtainedwhenusingtheproceduresgivenin
control to test against the neutralizing effect. Even more effective would
these methods apply only to the species which are used for the
be diluting the challenged adhesive specimen with Letheen broth and
testing. The test species listed in Section 9 are frequently used running Letheen agar pour plates. See Note 1 and Note 3. Extending the
incubation period of negative plates would be another safeguard. To
by laboratories to test for antimicrobial properties, but they are
neutralize thiazoline-based preservatives, 10 to 50 ppm of sodium
nottheonlyoneswhichcouldbeused.Selectionofthespecies
thioglycolate can be added to the medium.
touseforthesetestmethodsrequiresinformedjudgmentbythe
testing laboratory or by the party requesting the tests. It is also
5.6 These test methods are dependent upon the physiologi-
important that species which commonly attack adhesives be
cal action of living microorganisms under a reported set of
used. See 9.4.
conditions. Conclusions about the resistance of the test adhe-
sive to microbiological attack can be drawn by comparing the
5.5 The presence of an active biocide carried over from the
resultstosimultaneouslyruncontrolsofknownresistance.See
adhesive specimen to the agar could have an inhibiting effect
on the growth of microorganisms, resulting in no growth X5.2 for statements regarding test repeatability.
D4783 − 01 (2021)
6. Apparatus 7.14 Tryptone Glucose Extract Agar, dehydrated (Difco or
equivalent).
6.1 In addition to the standard equipment found in any fully
equipped microbiological laboratory, the following items are
7.15 Yeast Malt Peptone Glucose Agar (YMPGA), dehy-
sometimes needed: drated (Difco or equivalent).
6.1.1 Autoclave, capable of producing 103 kPa of steam
pressureat121°C(250°F)andmaintainingitforaminimumof
8. Precautions
15 min.
8.1 These test methods employ live cultures of bacteria,
6.1.2 Cell Counting Chamber, Petroff-Hausser, cell depth
fungi,andyeast,someofwhicharecapableofcausingdisease,
0.02 mm (or equivalent).
and others allergic reaction in some humans. In addition to
6.1.3 Bottles, Screwcap, approximately 375 mL, Boston
other precautions, assign laboratory personnel trained in cor-
Rounds of flint glass. Mold-A-7232-D, Finish 28-400, and
rect microbiological techniques to run these tests. Use proper
Black Artmold Caps BM-8041, Size 28-400, with rubber ring
microbiological procedures in order to prevent contamination
liners fastened to caps with steamproof adhesive.
of the cultures or of the work area. Clean and sterilize in an
6.1.4 Constant Temperature Chamber, capable of being
approved manner all spills and all equipment coming into
maintained at 35 6 0.5°C (95 6 1°F
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.