ASTM E168-99(2004)
(Practice)Standard Practices for General Techniques of Infrared Quantitative Analysis
Standard Practices for General Techniques of Infrared Quantitative Analysis
SCOPE
1.1 These practices cover the techniques most often used in infrared quantitative analysis. Practices associated with the collection and analysis of data on a computer are included as well as practices that do not use a computer.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific hazard statements appear in Section 6 and Note A4.7, Note A4.11, and Note A5.6.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:E168–99 (Reapproved 2004)
Standard Practices for
General Techniques of Infrared Quantitative Analysis
This standard is issued under the fixed designation E168; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 4. Significance and Use
1.1 These practices cover the techniques most often used in 4.1 These practices are intended for all infrared spectrosco-
infrared quantitative analysis. Practices associated with the pists. For novices, these practices will serve as an overview of
collection and analysis of data on a computer are included as preparation, operation, and calculation techniques. For experi-
well as practices that do not use a computer. enced persons, these practices will serve as a review when
1.2 This standard does not purport to address all of the seldom-used techniques are needed.
safety concerns, if any, associated with its use. It is the
5. Apparatus
responsibility of the user of this standard to establish appro-
5.1 The infrared techniques described here assume that the
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use. Specific hazard equipmentisofatleasttheusualcommercialqualityandmeets
the standard specifications of the manufacturer. For dispersive
statementsappearinSection6andNoteA4.7,NoteA4.11,and
Note A5.6. instruments, also refer to Practice E932. For Fourier Trans-
form and dispersive instruments, also refer to Practices E1421
2. Referenced Documents
and E932 respectively, and for microanalysis with these
2.1 ASTM Standards: instruments see Practice E334.
E131 Terminology Relating to Molecular Spectroscopy 5.2 In developing a spectroscopic method, it is the respon-
E334 Practice for General Techniques of Infrared Mi- sibilityoftheoriginatortodescribetheinstrumentationandthe
croanalysis performance required to duplicate the repeatability and bias of
E932 Practice for Describing and Measuring Performance a method. It is necessary to specify this performance in terms
of Dispersive Infrared Spectrometers that can be used by others in applications of the method.
E1252 Practice for General Techniques for Qualitative
6. Hazards
Infrared Analysis
6.1 Users of these practices must be aware that there are
E1421 PracticeforDescribingandMeasuringPerformance
of Fourier Transform Infrared (FT-IR) Spectrometers: inherent dangers associated with the use of electrical instru-
mentation, infrared cells, solvents, and other chemicals, and
Level Zero and Level One Tests
E1655 Practices for Infrared Multivariate Quantitative thatthesepracticescannotandwillnotsubstituteforapractical
knowledge of the instrument, cells, and chemicals used in a
Analysis
particular analysis.
3. Terminology
7. Considerations for Quantitative Infrared
3.1 For definitions of terms and symbols, refer toTerminol-
Measurements
ogy E131.
7.1 Quantitative infrared analysis is commonly done with
grating, filter, prism, or interferometer instruments. The fol-
These practices are under the jurisdiction of ASTM Committee E13 on
lowing guidelines for setting up an analytical procedure are
Molecular Spectroscopy and are the direct responsibility of Subcommittee E13.03
appropriate:
on Infrared Spectroscopy.
7.1.1 Always operate the instrument in the most stable and
Current edition approved Feb. 1, 2004. Published March 2004. Originally
approved in 1964. Last previous edition approved in 1999 as E168–99.
reproducible conditions attainable. This includes instrument
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
warm-up time, sample temperature equilibration, and exact
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
reproduction of instrument performance tests for both stan-
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. dardsandsamples.Aftercalibration,useequivalentsettingsfor
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
E168–99 (2004)
analyses. For all infrared instruments, refer to the manufactur- 7.1.7 Use spectral regions offering the most information on
er’s recommendations for the instrument settings. After cali- the analyte. Select analytical wavenumbers where the compo-
bration, use these same settings for analysis. nent has a relatively large absorptivity. In addition, other
7.1.2 The absorbance values at analytical wavenumbers analytes should have minimal effect on the measured absor-
should fall within the acceptably accurate range of the particu- bance.
larspectrometerused.Ingeneral,asingleabsorbancemeasure- 7.1.8 The performance of the spectrometer should be suffi-
ment will have the best signal-to-noise ratio when it is in the ciently good to give adequate linearity of response for the
range from 0.3 to 0.8 absorbance units (AU) (1). The desiredrangeofconcentrations.Thesignal-to-noiseratio,S/N,
sensitivity of Fourier transform (FT-IR) spectrometers is such should be acceptable for the desired precision.
that lower absorbance values can be used quite effectively, 7.1.9 Select analytical wavenumbers such that the linearity
provided that the baseline can be estimated accurately (see of the absorbance-concentration relationship is least affected
Section 12). Absorbances greater than 0.8 AU should be by molecular interaction, dispersion in refractive index, and
avoided wherever possible because of the possibility of spectrometer nonlinearity.
instrumentally-causednon-linearity,bothfordispersive(2)and
8. Theory for a Single-Compound Analysis
FT-IR (3,4) spectrometers. Variation of the concentration and
samplepathlengthcanbeusedtoadjustabsorbancevaluesinto
8.1 Quantitative spectrometry is based on the Beer-
theoptimumrange.Whenmultiplecomponentsaredetermined
Bouguer-Lambert(henceforthreferredtoasBeer’s)law,which
inaparticularsample,itisacceptabletouseabsorbancevalues
is expressed for the one component case as:
outside the optimum range, (5) however, absorbances greater
A 5 abc (1)
than1.5AUshouldbeavoided (2-4).Weakerabsorptionbands
of high concentration components may be selected to provide
where:
absorbance values within the optimal range.
A = absorbance of the sample at a specified wavenumber,
a = absorptivity of the component at this wavenumber,
7.1.3 The most accurate analytical methods are imple-
b = sample path length, and
mented with samples in solution. With liquid samples that are
c = concentration of the component.
notexceptionallyviscous,bestresultsareobtainedifthecellis
Since spectrometers measure transmittance, T, of the radia-
not moved after the first sample is introduced into the instru-
tion through a sample, it is necessary to convert T to A as
ment (the fixed-cell method). The reason is that sample cell
follows:
position is difficult to reproduce accurately by insertion into
typical cell holders. Suitable fittings and tubes can be attached
P
A52log T52log (2)
to the cell to allow sample changing in a flow-through manner.
P
When it is not practical to use a flow-through cell, the cell
where:
shouldfittightlyintheholdersothatlateralandtiltingmotions
P = input radiant power at the sample, and
are restricted.
P = radiant power transmitted through the sample.
7.1.4 Unless there is reason to suspect deposition on or
contamination of the cell from the samples, it is generally
9. Calibration for a Single-Component Determination
preferabletowashoutthecurrentsamplewiththenextsample,
9.1 Proper sample preparation is essential to quantitative
if sufficient sample is available.The volume of sample used to
analysis. See Annex A4.
flushthecellshouldbeatleastfivetimes(andpreferablymore,
9.1.1 Quantitative analysis has two distinct parts: calibra-
for example, 20 times) the volume between the sample inlet
tion and analysis. For a simple one-component analysis, select
and cell exit points.
an appropriate solvent that is essentially free from interfering
7.1.5 For some bands, the wavenumber of the maximum
absorptions at the analytical wavenumber.
absorbance changes as a function of concentration. Similarly,
9.1.2 For calibration, measure the absorbances, A,ofthe
the position of the baseline points may change with concen-
analyte solutions at several known concentrations, c. Absorp-
tration. Selection of baseline points must be done carefully to
tivities, a, are then calculated, using Eq 1 with the baseline
accountfortheshiftoftheabsorbancemaximum.Thequestion
corrections as described in Sections 12-14. Alternatively, the
arises whether it is preferable to measure absorbances at fixed
absorbances, A,ofasinglesolutioninseveralcellsofdifferent,
wavenumber locations or at the observed maximum of the
butaccuratelyknown,pathlengthsmaybemeasured;however,
analytical band. The best approach is empirical testing of both
interaction effects will not be elucidated in this fashion.
the fixed point and the tracking methods of evaluation.
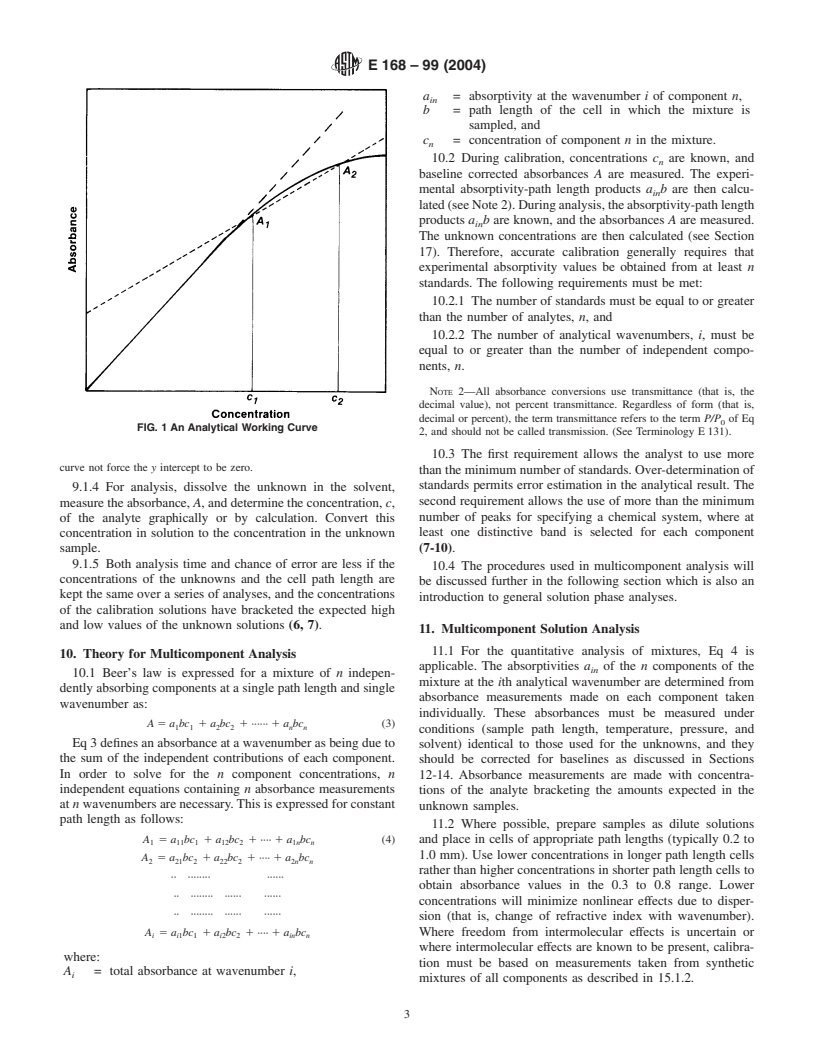
9.1.3 Calculatetheaverageoftheseveral avaluesforfuture
7.1.6 Whenever possible, working directly in absorbance is
use, or draw an analytical working curve by graphing absor-
preferable. That is, either the instrument or associated data
bance versus concentration for a constant path length as
processor makes the necessary conversion from transmittance
demonstrated in Fig. 1. Use the linear part of the curve to
to absorbance. If spectra cannot be obtained in absorbance,
calculate a.Thecalculationof awherecurvatureispresentwill
thenEqA12.1andA12.2inAnnexA12canbeusedtoconvert
be discussed in 18.1 and 18.2.
the data.
NOTE 1—Inpractice,thecalibrationcurvemaynothavea yinterceptof
zero.Thiscouldbeduetoavarietyoffactorsincluding,butnotlimitedto,
incompletely resolved analyte bands, reflection losses, and solvent inter-
Theboldfacenumbersinparenthesesrefertothelistofreferencesattheendof
these practices. ferences. It is important that the method used to calculate the calibration
E168–99 (2004)
a = absorptivity at the wavenumber i of component n,
in
b = path length of the cell in which the mixture is
sampled, and
c = concentration of component n in the mixture.
n
10.2 During calibration, concentrations c are known, and
n
baseline corrected absorbances A are measured. The experi-
mental absorptivity-path length products a b are then calcu-
in
lated(seeNote2).Duringanalysis,theabsorptivity-pathlength
products a b are known, and the absorbances A are measured.
in
The unknown concentrations are then calculated (see Section
17). Therefore, accurate calibration generally requires that
experimental absorptivity values be obtained from at least n
standards. The following requirements must be met:
10.2.1 The number of standards must be equal to or greater
than the number of analytes, n, and
10.2.2 The number of analytical wavenumbers, i, must be
equal to or greater than the number of independent compo-
nents, n.
NOTE 2—All absorbance conversions use transmittance (that is, the
decimal value), not percent transmittance. Regardless of form (that is,
decimal or percent), the term transmittance refers to the term P/P of Eq
FIG. 1 An Analytical Working Curve
2, and should not be called transmission. (See Terminology E131).
10.3 The first requirement allows the analyst to use more
curve not force the y intercept to be zero.
thantheminimumnumberofstandards.Over-determinationof
standards permits error estimation in the analytical result. The
9.1.4 For analysis, dissolve the unknown in the solvent,
second requirement allows the use of more than the minimum
measuretheabsorbance, A,anddeterminetheconcentration, c,
number of peaks for specifying a chemical system, where at
of the analyte graphically or by calculation. Convert this
concentration in solution to the concentration in the unknown least one distinctive band is selected for each component
(7-10).
sample.
9.1.5 Both analysis time and chance of error are less if the
10.4 The procedures used in multicomponent analysis will
concentrations of the unknowns and the cell path length are
be discussed further in the following section which is also an
kept the same over a series of analyses, and the concentrations
introduction to general solution phase analyses.
of the calibration solutions have bracketed the expected high
and low values of the unknown solutions (6, 7).
11. Multicomponent Solution Analysis
11.1 For the quantitative analysis of mixtures, Eq 4 is
10. Theory for Multicomponent Analysis
applicable. The absorptivities a of the n components of the
in
10.1 Beer’s law is expressed for a mixture of n indepen-
mixture at the ith analytical wavenumber are determined from
dentlyabsorbingcomponentsatasinglepathlengthandsingle
absorbance measurements made on each component taken
wavenumber as:
individually. These absorbances must be measured under
A 5 a bc 1 a bc 1······ 1 a bc (3)
1 1 2 2 n n
conditions (sample path length, temperature, pressure, and
Eq3definesanabsorbanceatawavenumberasbeingdueto solvent) identical to those used for the unknowns, and they
the sum of the independent contributions of each component.
should be corrected for baselines as discussed in Sections
In order to solve for the n component concentrations, n
12-14. Absorbance measurements are made with concentra-
independent equations containing n absorbance measurements
tions of the analyte bracketing the amounts expected in the
at nwavenumbersarenecessary.Thisisexpressedforconstant
unknown samples.
path length as follows:
11.2 Where possible, prepare samples as dilute solutions
A 5 a bc 1 a bc 1···· 1 a bc (4)
and place in cells of appropriate path lengths (typically 0.2 to
1 11 1 12 2 1n n
1.0 mm). Use lower concentrations in longer path length cells
A 5 a bc 1 a bc 1···· 1 a bc
2 21 2 22 2 2n n
rather than higher concentrations in shorter path length cells to
·· ········ ······
obtain absorbance values in the 0.3 to 0.8 range. Lower
·· ········ ······ ······
concentrations will minimize nonlinear effects due to disper-
·· ········ ······ ······
sion (that is, change of refractive index with wavenumber).
A 5 a bc 1 a bc 1···· 1 a bc Where freedom from intermolecular effects is uncertain or
i i1 1 i2 2 in n
where intermolecular effects are known to be present, calibra-
where:
tion must be based on measurements taken from synthetic
A = total absorbance at wavenumber i,
i
mixtures of all components as described in 15.1.2.
E168–99 (2004)
11.3 Dissolve a known weight of a pure component in a
suitable in
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.