ASTM E168-16(2023)
(Practice)Standard Practices for General Techniques of Infrared Quantitative Analysis
Standard Practices for General Techniques of Infrared Quantitative Analysis
SIGNIFICANCE AND USE
4.1 These practices are intended for all infrared spectroscopists. For novices, these practices will serve as an overview of preparation, operation, and calculation techniques. For experienced persons, these practices will serve as a review when seldom-used techniques are needed.
SCOPE
1.1 These practices cover the techniques most often used in infrared quantitative analysis. Practices associated with the collection and analysis of data on a computer are included as well as practices that do not use a computer.
1.2 This practice does not purport to address all of the concerns associated with developing a new quantitative method. It is the responsibility of the developer to ensure that the results of the method fall in the desired range of precision and bias.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use. Specific hazard statements appear in Section 6, Note A4.7, Note A4.11, and Note A5.6.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: E168 − 16 (Reapproved 2023)
Standard Practices for
General Techniques of Infrared Quantitative Analysis
This standard is issued under the fixed designation E168; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
1. Scope E932PracticeforDescribingandMeasuringPerformanceof
Dispersive Infrared Spectrometers
1.1 These practices cover the techniques most often used in
E1252Practice for General Techniques for Obtaining Infra-
infrared quantitative analysis. Practices associated with the
red Spectra for Qualitative Analysis
collection and analysis of data on a computer are included as
E1421Practice for Describing and Measuring Performance
well as practices that do not use a computer.
of Fourier Transform Mid-Infrared (FT-MIR) Spectrom-
1.2 This practice does not purport to address all of the
eters: Level Zero and Level One Tests
concerns associated with developing a new quantitative
E1655 Practices for Infrared Multivariate Quantitative
method. It is the responsibility of the developer to ensure that
Analysis
the results of the method fall in the desired range of precision
and bias.
3. Terminology
1.3 The values stated in SI units are to be regarded as
3.1 For definitions of terms and symbols, refer toTerminol-
standard. No other units of measurement are included in this
ogy E131.
standard.
4. Significance and Use
1.4 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the 4.1 These practices are intended for all infrared spectrosco-
responsibility of the user of this standard to establish appro- pists. For novices, these practices will serve as an overview of
priate safety, health, and environmental practices and deter- preparation, operation, and calculation techniques. For experi-
mine the applicability of regulatory limitations prior to use. enced persons, these practices will serve as a review when
Specific hazard statements appear in Section 6, Note A4.7, seldom-used techniques are needed.
Note A4.11, and Note A5.6.
5. Apparatus
1.5 This international standard was developed in accor-
dance with internationally recognized principles on standard-
5.1 The infrared techniques described here assume that the
ization established in the Decision on Principles for the
equipmentisofatleasttheusualcommercialqualityandmeets
Development of International Standards, Guides and Recom-
the standard specifications of the manufacturer. For dispersive
mendations issued by the World Trade Organization Technical
instruments,alsorefertoPracticeE932.ForFourierTransform
Barriers to Trade (TBT) Committee.
and dispersive instruments, also refer to Practices E1421 and
E932 respectively, and for microanalysis with these instru-
2. Referenced Documents
ments see Practice E334.
2.1 ASTM Standards:
5.2 In developing a spectroscopic method, it is the respon-
E131Terminology Relating to Molecular Spectroscopy
sibilityoftheoriginatortodescribetheinstrumentationandthe
E334Practice for General Techniques of Infrared Micro-
performance required to duplicate the precision and bias of a
analysis
method. It is necessary to specify this performance in terms
that can be used by others in applications of the method.
These practices are under the jurisdiction of ASTM Committee E13 on
6. Hazards
Molecular Spectroscopy and Separation Science and are the direct responsibility of
Subcommittee E13.03 on Infrared and Near Infrared Spectroscopy.
6.1 Users of these practices must be aware that there are
Current edition approved Jan. 1, 2023. Published January 2023. Originally
inherent dangers associated with the use of electrical
approved in 1964. Last previous edition approved in 2016 as E168–16. DOI:
10.1520/E0168-16R23.
instrumentation, infrared cells, solvents, and other chemicals,
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
and that these practices cannot and will not substitute for a
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
practical knowledge of the instrument, cells, and chemicals
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. used in a particular analysis.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E168 − 16 (2023)
7. Considerations for Quantitative Infrared accountfortheshiftoftheabsorbancemaximum.Thequestion
Measurements arises whether it is preferable to measure absorbances at fixed
wavenumber locations or at the observed maximum of the
7.1 Quantitative infrared analysis is commonly done with
analytical band. The best approach is empirical testing of both
grating, filter, prism, or interferometer instruments. The fol-
the fixed point and the tracking methods of evaluation.
lowing guidelines for setting up an analytical procedure are
7.1.6 Whenever possible, working directly in absorbance is
appropriate:
preferable. That is, either the instrument or associated data
7.1.1 Always operate the instrument in the most stable and
processor makes the necessary conversion from transmittance
reproducible conditions attainable. This includes instrument
to absorbance. If spectra cannot be obtained in absorbance,
warm-up time, sample temperature equilibration, and exact
thenEqA12.1andA12.2inAnnexA12canbeusedtoconvert
reproduction of instrument performance tests for both stan-
the data.
dardsandsamples.Aftercalibration,useequivalentsettingsfor
7.1.7 Use spectral regions offering the most information on
analyses. For all infrared instruments, refer to the manufactur-
the analyte. Select analytical wavenumbers where the compo-
er’s recommendations for the instrument settings. After
nent has a relatively large absorptivity. In addition, other
calibration, use these same settings for analysis.
analytes should have minimal effect on the measured absor-
7.1.2 The absorbance values at analytical wavenumbers
bance.
should fall within the acceptably accurate range of the particu-
7.1.8 The performance of the spectrometer should be suffi-
larspectrometerused.Ingeneral,asingleabsorbancemeasure-
ciently good to give adequate linearity of response for the
ment will have the best signal-to-noise ratio when it is in the
3 desiredrangeofconcentrations.Thesignal-to-noiseratio,S/N,
range from 0.3 to 0.8 absorbance units (AU) (1). The
should be acceptable for the desired precision.
sensitivity of Fourier transform (FT-IR) spectrometers is such
7.1.9 Select analytical wavenumbers such that the linearity
that lower absorbance values can be used quite effectively,
of the absorbance-concentration relationship is least affected
provided that the baseline can be estimated accurately (see
by molecular interaction, dispersion in refractive index, and
Section 12). Absorbances greater than 0.8 AU should be
spectrometer nonlinearity.
avoided wherever possible because of the possibility of
instrumentally-causednon-linearity,bothfordispersive (2)and
8. Theory for a Single-Compound Analysis
FT-IR (3,4) spectrometers. Variation of the concentration and
samplepathlengthcanbeusedtoadjustabsorbancevaluesinto 8.1 Quantitative spectrometry is based on the Beer-
theoptimumrange.Whenmultiplecomponentsaredetermined
Bouguer-Lambert(henceforthreferredtoasBeer’s)law,which
inaparticularsample,itisacceptabletouseabsorbancevalues is expressed for the one component case as:
outside the optimum range, (5) however, absorbances greater
A 5 abc (1)
than1.5AUshouldbeavoided (2-4).Weakerabsorptionbands
where:
of high concentration components may be selected to provide
absorbance values within the optimal range. A = absorbance of the sample at a specified wavenumber,
7.1.3 The most accurate analytical methods are imple- a = absorptivity of the component at this wavenumber,
b = sample path length, and
mented with samples in solution. With liquid samples that are
c = concentration of the component.
notexceptionallyviscous,bestresultsareobtainedifthecellis
not moved after the first sample is introduced into the instru-
Since spectrometers measure transmittance, T, of the radia-
ment (the fixed-cell method). The reason is that sample cell
tion through a sample, it is necessary to convert T to A as
position is difficult to reproduce accurately by insertion into
follows:
typical cell holders. Suitable fittings and tubes can be attached
P
to the cell to allow sample changing in a flow-through manner.
A52logT52log (2)
P
When it is not practical to use a flow-through cell, the cell
shouldfittightlyintheholdersothatlateralandtiltingmotions where:
are restricted.
P = input radiant power at the sample, and
7.1.4 Unless there is reason to suspect deposition on or
P = radiant power transmitted through the sample.
contamination of the cell from the samples, it is generally
preferabletowashoutthecurrentsamplewiththenextsample,
9. Calibration for a Single-Component Determination
if sufficient sample is available.The volume of sample used to
9.1 Proper sample preparation is essential to quantitative
flushthecellshouldbeatleastfivetimes(andpreferablymore,
analysis. See Annex A4.
for example, 20 times) the volume between the sample inlet
9.1.1 Quantitative analysis has two distinct parts: calibra-
and cell exit points.
tion and analysis. For a simple one-component analysis, select
7.1.5 For some bands, the wavenumber of the maximum
an appropriate solvent that is essentially free from interfering
absorbance changes as a function of concentration. Similarly,
absorptions at the analytical wavenumber.
the position of the baseline points may change with concen-
9.1.2 For calibration, measure the absorbances, A,ofthe
tration. Selection of baseline points must be done carefully to
analyte solutions at several known concentrations, c.
Absorptivities, a, are then calculated, using Eq 1 with the
baseline corrections as described in Sections 12–14.
Theboldfacenumbersinparenthesesrefertothelistofreferencesattheendof
these practices. Alternatively,theabsorbances, A,ofasinglesolutioninseveral
E168 − 16 (2023)
cells of different, but accurately known, path lengths may be independent equations containing n absorbance measurements
measured;however,interactioneffectswillnotbeelucidatedin at nwavenumbersarenecessary.Thisisexpressedforconstant
this fashion. path length as follows:
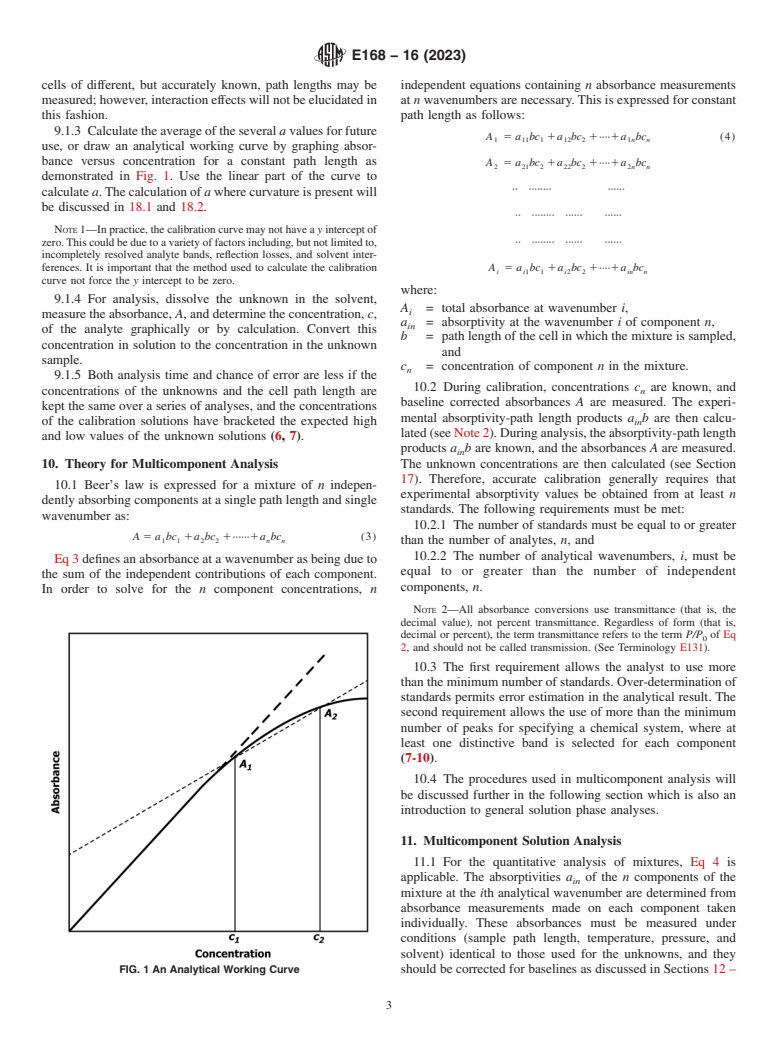
9.1.3 Calculatetheaverageoftheseveral avaluesforfuture
A 5 a bc 1a bc 1····1a bc (4)
1 11 1 12 2 1n n
use, or draw an analytical working curve by graphing absor-
bance versus concentration for a constant path length as A 5 a bc 1a bc 1····1a bc
2 21 2 22 2 2n n
demonstrated in Fig. 1. Use the linear part of the curve to
·· ········ ······
calculate a.Thecalculationof awherecurvatureispresentwill
be discussed in 18.1 and 18.2.
·· ········ ······ ······
NOTE1—Inpractice,thecalibrationcurvemaynothavea yinterceptof
·· ········ ······ ······
zero.Thiscouldbeduetoavarietyoffactorsincluding,butnotlimitedto,
incompletely resolved analyte bands, reflection losses, and solvent inter-
ferences. It is important that the method used to calculate the calibration A 5 a bc 1a bc 1····1a bc
i i1 1 i2 2 in n
curve not force the y intercept to be zero.
where:
9.1.4 For analysis, dissolve the unknown in the solvent,
A = total absorbance at wavenumber i,
i
measuretheabsorbance, A,anddeterminetheconcentration, c,
a = absorptivity at the wavenumber i of component n,
in
of the analyte graphically or by calculation. Convert this
b = path length of the cell in which the mixture is sampled,
concentration in solution to the concentration in the unknown
and
sample.
c = concentration of component n in the mixture.
n
9.1.5 Both analysis time and chance of error are less if the
10.2 During calibration, concentrations c are known, and
n
concentrations of the unknowns and the cell path length are
baseline corrected absorbances A are measured. The experi-
kept the same over a series of analyses, and the concentrations
mental absorptivity-path length products a b are then calcu-
in
of the calibration solutions have bracketed the expected high
lated(seeNote2).Duringanalysis,theabsorptivity-pathlength
and low values of the unknown solutions (6, 7).
products a b are known, and the absorbances A are measured.
in
10. Theory for Multicomponent Analysis The unknown concentrations are then calculated (see Section
17). Therefore, accurate calibration generally requires that
10.1 Beer’s law is expressed for a mixture of n indepen-
experimental absorptivity values be obtained from at least n
dentlyabsorbingcomponentsatasinglepathlengthandsingle
standards. The following requirements must be met:
wavenumber as:
10.2.1 The number of standards must be equal to or greater
A 5 a bc 1a bc 1······1a bc (3)
1 1 2 2 n n
than the number of analytes, n, and
10.2.2 The number of analytical wavenumbers, i, must be
Eq3definesanabsorbanceatawavenumberasbeingdueto
equal to or greater than the number of independent
the sum of the independent contributions of each component.
components, n.
In order to solve for the n component concentrations, n
NOTE 2—All absorbance conversions use transmittance (that is, the
decimal value), not percent transmittance. Regardless of form (that is,
decimal or percent), the term transmittance refers to the term P/P of Eq
2, and should not be called transmission. (See Terminology E131).
10.3 The first requirement allows the analyst to use more
thantheminimumnumberofstandards.Over-determinationof
standards permits error estimation in the analytical result. The
second requirement allows the use of more than the minimum
number of peaks for specifying a chemical system, where at
least one distinctive band is selected for each component
(7-10).
10.4 The procedures used in multicomponent analysis will
be discussed further in the following section which is also an
introduction to general solution phase analyses.
11. Multicomponent Solution Analysis
11.1 For the quantitative analysis of mixtures, Eq 4 is
applicable. The absorptivities a of the n components of the
in
mixture at the ith analytical wavenumber are determined from
absorbance measurements made on each component taken
individually. These absorbances must be measured under
conditions (sample path length, temperature, pressure, and
solvent) identical to those us
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.