ASTM D2187-94(2004)
(Test Method)Standard Test Methods for Physical and Chemical Properties of Particulate Ion-Exchange Resins
Standard Test Methods for Physical and Chemical Properties of Particulate Ion-Exchange Resins
SIGNIFICANCE AND USE
The ionic form of an ion-exchange material affects both its equivalent mass and its equilibrium water content. These in turn influence the numerical values obtained in exchange capacity determinations, in density measurements, and in the size of the particles. To provide a uniform basis for comparison, therefore, the sample should be converted to a known ionic form before analysis. This procedure provides for the conversion of cation-exchange materials to the sodium form and anion-exchange materials to the chloride form prior to analysis. These forms are chosen since they permit samples to be weighed and dried without concern for air contamination or decomposition. If other ionic forms are used this fact should be noted in reporting the results.
SCOPE
1.1 These test methods cover the determination of the physical and chemical properties of ion-exchange resins when used for the treatment of water. They are intended for use in testing both new and used materials.
1.2 The values stated in SI units are to be regarded as the standard. The inch-pound units given in parentheses are for information only.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific precautionary statements are given in Note 1.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D 2187–94 (Reapproved 2004)
Standard Test Methods for
Physical and Chemical Properties of Particulate Ion-
Exchange Resins
This standard is issued under the fixed designation D 2187; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
1.1 These test methods cover the determination of the 2.1 ASTM Standards:
physical and chemical properties of ion-exchange resins when D 1129 Terminology Relating to Water
used for the treatment of water. They are intended for use in D 1193 Specification for Reagent Water
testingbothnewandusedmaterials.Thefollowingthirteentest D 1293 Test Methods for pH of Water
methods are included: D 2687 Practices for Sampling Particulate Ion-Exchange
Materials
D 2777 Practice for Determination of Precision and Bias of
Sections
Test MethodA—Pretreatment 6-10
Applicable Methods of Committee D19 on Water
Test Method B—Water Retention Capacity 11-17
E11 Specification for Wire Cloth and Sieves for Testing
Test Method C—Backwashed and Settled Density 18-24
Purposes
Test Method D—Particle Size Distribution 25-32
Test Method E—Salt-Splitting Capacity of Cation- 33-41
Exchange Resins
3. Terminology
Test Method F—Total Capacity of Cation-Exchange 42-50
3.1 Definitions—For definitions of terms used in these test
Resins
Test Method G—Percent Regeneration of Hydrogen- 51-58
methods refer to Terminology D1129.
Form Cation-Exchange Resins
3.2 Definitions of Terms Specific to This Standard:
Test Method H—Total and Salt-Splitting Capacity of 59-66
3.2.1 anion-exchange material—an ion-exchange material
Anion-Exchange Resins
Test Method I—Percent Regeneration ofAnion 67-75
capable of the reversible exchange of negatively charged ions.
Exchange Resins
3.2.2 cation-exchange material—an ion-exchange material
Test Method J—Ionic Chloride Content ofAnion- 76-83
capable of the reversible exchange of positively charged ions.
Exchange Resins
Test Method K—Carbonate Content ofAnion- 84-91
3.2.3 ion-exchange resin—asyntheticorganicion-exchange
Exchange Resins
material.
Test Method L—Sulfate Content ofAnion Exchange 92-99
Resins 3.2.4 mixed bed—a physical mixture of anion-exchange
Test Method M—TotalAnion Capacity ofAnion- 100-108
material and cation-exchange material.
Exchange Resins
4. Reagents
1.2 The values stated in SI units are to be regarded as the
standard. The inch-pound units given in parentheses are for
4.1 Purity of Reagents—Reagent grade chemicals shall be
information only.
used in all tests. Unless otherwise indicated, it is intended that
1.3 This standard does not purport to address all of the
all reagents shall conform to the specifications of the Commit-
safety concerns, if any, associated with its use. It is the
tee onAnalytical Reagents of theAmerican Chemical Society,
responsibility of the user of this standard to establish appro-
where such specifications are available. Other grades may be
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use. Specific precau-
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
tionary statements are given in Note 1.
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website.
Reagent Chemicals, American Chemical Society Specifications, American
These test methods are under the jurisdiction of ASTM Committee D19 on Chemical Society, Washington, DC. For suggestions on the testing of reagents not
Water and are the direct responsibility of Subcommittee D19.08 on Membranes and listed by the American Chemical Society, see Analar Standards for Laboratory
Ion Exchange Materials. Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
Current edition approved June 1, 2004. Published June 2004. Originally and National Formulary, U.S. Pharmaceutical Convention, Inc. (USPC), Rockville,
approved in 1963. Last previous edition approved in 1998 as D2187–94 (1998). MD.
Copyright ©ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA19428-2959, United States.
D 2187–94 (2004)
used, provided it is first ascertained that the reagent is of
sufficiently high purity to permit its use without lessening the
accuracy of the determination.
4.2 Purity of Water— Unless otherwise indicated, refer-
ences to water shall be understood to mean Type IV reagent
water described in Specification D1193.
5. Sampling
5.1 Obtain a representative sample of the ion-exchange
resin in accordance with Practices D2687.
5.2 A minimum sample size of 1 L is recommended for a
complete testing program.
TEST METHOD A—PRETREATMENT
6. Scope
6.1 This test method covers the conversion of ion-exchange
resins to a known ionic form and is intended for application to
both new and used material.
7. Significance and Use
7.1 The ionic form of an ion-exchange material affects both
its equivalent mass and its equilibrium water content. These in
turn influence the numerical values obtained in exchange
capacity determinations, in density measurements, and in the
size of the particles. To provide a uniform basis for compari-
FIG. 1 Typical Arrangement of Apparatus for Pretreatment of Ion-
son, therefore, the sample should be converted to a known
Exchange Materials
ionic form before analysis. This procedure provides for the
conversion of cation-exchange materials to the sodium form
8.2.4 Vacuum Pump, capable of creating a pressure differ-
and anion-exchange materials to the chloride form prior to
ential 40 mm Hg below atmospheric pressure.
analysis. These forms are chosen since they permit samples to
be weighed and dried without concern for air contamination or 9. Reagents
decomposition.Ifotherionicformsareusedthisfactshouldbe
9.1 Hydrochloric Acid (1 + 9)—Carefully pour 100 mL of
noted in reporting the results.
hydrochloric acid (HCl, sp gr 1.19) into 900 mL of water,
stirring constantly. Cool to 25 6 5°C.
8. Apparatus
9.2 Sodium Chloride Solution (100 g/L)—Dissolve 100.0 g
8.1 Pretreatment Apparatus (See Fig. 1): of sodium chloride (NaCl) in 800 mL of water and dilute to 1
8.1.1 Column, transparent, vertically-supported, 25 6 2.5 L.
mm (1.0 6 0.1 in.) inside diameter and approximately 1500 9.3 Sodium Chloride Solution (240 g/L)—Dissolve240gof
mm(60in.)long.Thebottomofthecolumnshallbeclosedand sodium chloride (NaCl) in 800 mL of water and dilute to 1 L.
provided with an outlet of approximately 6-mm inside diam- 9.4 Sodium Hydroxide Solution (40 g/L)—Dissolve 40.0 g
eter. Connections shall be provided at top and bottom for
of sodium hydroxide (NaOH) in 800 mL of water. Cool and
admissionandremovalofsolutionsasdescribedinSection10. dilute to 1 L.
Adequate means for measuring and regulating flow shall be
9.5 Thymol Blue Indicator Solution—Dissolve 0.1 g of
provided. Calibrate the column in such a manner that the thymol blue (thymol sulfonphthalein) in 10.75 mL of 0.02 N
volumereadingsrequiredbythemethodcanbemade.Makeall
NaOH solution. Dilute to 250 mL with water.
measurements at 25 6 5°C. 9.6 Tropaeolin O Indicator Solution—Dissolve 0.10 g of
8.1.2 Support, for the sample, so designed that the distance
tropaeolin O (p-benzene-sulfonic acid-azoresorcinol) in 50 mL
from the sample to the column outlet is at least 50 mm. of water and dilute to 100 mL in a volumetric flask.
Suggested supports are corrosion-resistant screen or porous
10. Procedure
plate.
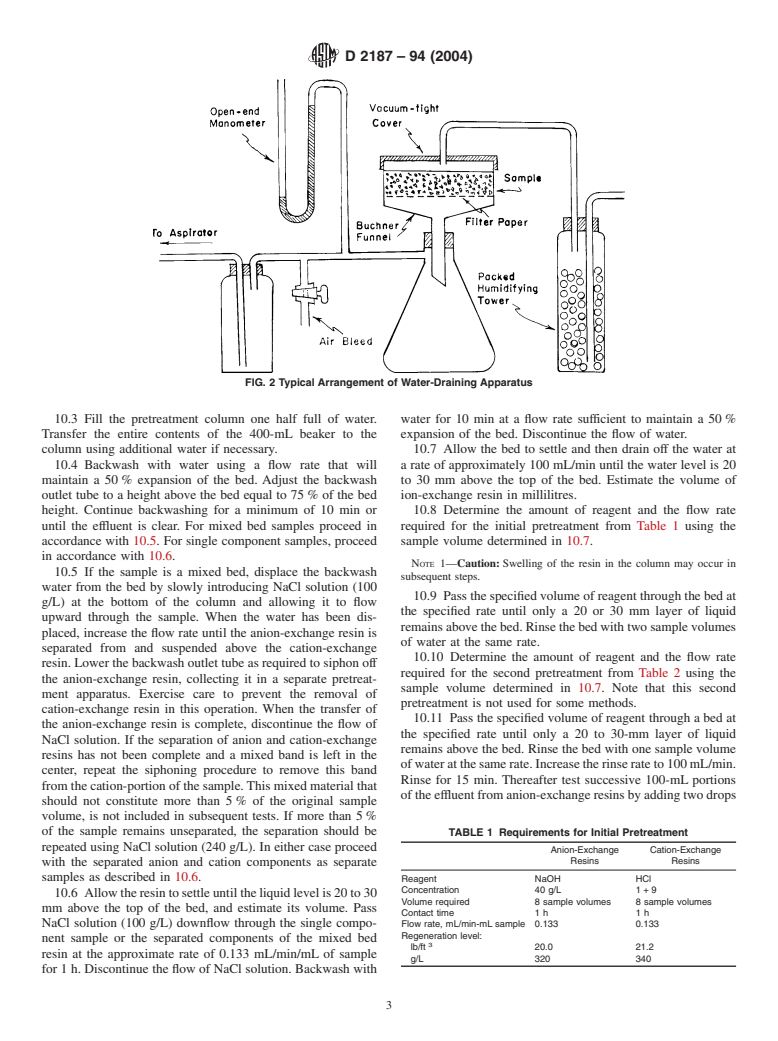
8.2 Draining Apparatus (Fig. 2): 10.1 Adjustthetemperatureofthewaterandallsolutionsto
8.2.1 Buchner-Type Funnel, containing a 125-mm filter be used in the procedure to 25 6 5°C and maintain this
paper and supported in a 1-L suction flask. temperature throughout the test.
8.2.2 Open-Arm Mercury Manometer, connected by a 10.2 Transfer the entire sample as received to a 2-L beaker
T-tube to a vacuum train. using water to rinse out the container.Adjust the water level to
8.2.3 Gas-Humidifying Tower, of at least 500 mL capacity, the sample level. Let stand a minimum of 1 h. Mix thoroughly
two thirds filled with glass beads or similar material. and transfer a representative sample to fill a 400-mL beaker.
D 2187–94 (2004)
FIG. 2 Typical Arrangement of Water-Draining Apparatus
10.3 Fill the pretreatment column one half full of water. water for 10 min at a flow rate sufficient to maintain a 50%
Transfer the entire contents of the 400-mL beaker to the expansion of the bed. Discontinue the flow of water.
column using additional water if necessary. 10.7 Allow the bed to settle and then drain off the water at
10.4 Backwash with water using a flow rate that will a rate of approximately 100 mL/min until the water level is 20
maintain a 50% expansion of the bed. Adjust the backwash to 30 mm above the top of the bed. Estimate the volume of
outlet tube to a height above the bed equal to 75% of the bed ion-exchange resin in millilitres.
height. Continue backwashing for a minimum of 10 min or 10.8 Determine the amount of reagent and the flow rate
until the effluent is clear. For mixed bed samples proceed in required for the initial pretreatment from Table 1 using the
accordance with 10.5. For single component samples, proceed sample volume determined in 10.7.
in accordance with 10.6.
NOTE 1—Caution:Swelling of the resin in the column may occur in
10.5 If the sample is a mixed bed, displace the backwash
subsequent steps.
water from the bed by slowly introducing NaCl solution (100
10.9 Passthespecifiedvolumeofreagentthroughthebedat
g/L) at the bottom of the column and allowing it to flow
the specified rate until only a 20 or 30 mm layer of liquid
upward through the sample. When the water has been dis-
remainsabovethebed.Rinsethebedwithtwosamplevolumes
placed, increase the flow rate until the anion-exchange resin is
of water at the same rate.
separated from and suspended above the cation-exchange
10.10 Determine the amount of reagent and the flow rate
resin.Lowerthebackwashoutlettubeasrequiredtosiphonoff
required for the second pretreatment from Table 2 using the
the anion-exchange resin, collecting it in a separate pretreat-
sample volume determined in 10.7. Note that this second
ment apparatus. Exercise care to prevent the removal of
pretreatment is not used for some methods.
cation-exchange resin in this operation. When the transfer of
10.11 Pass the specified volume of reagent through a bed at
the anion-exchange resin is complete, discontinue the flow of
the specified rate until only a 20 to 30-mm layer of liquid
NaCl solution. If the separation of anion and cation-exchange
remains above the bed. Rinse the bed with one sample volume
resins has not been complete and a mixed band is left in the
ofwateratthesamerate.Increasetherinserateto100mL/min.
center, repeat the siphoning procedure to remove this band
Rinse for 15 min. Thereafter test successive 100-mL portions
fromthecation-portionofthesample.Thismixedmaterialthat
oftheeffluentfromanion-exchangeresinsbyaddingtwodrops
should not constitute more than 5% of the original sample
volume, is not included in subsequent tests. If more than 5%
of the sample remains unseparated, the separation should be TABLE 1 Requirements for Initial Pretreatment
repeated using NaCl solution (240 g/L). In either case proceed
Anion-Exchange Cation-Exchange
Resins Resins
with the separated anion and cation components as separate
samples as described in 10.6.
Reagent NaOH HCl
Concentration 40 g/L 1+9
10.6 Allowtheresintosettleuntiltheliquidlevelis20to30
Volume required 8 sample volumes 8 sample volumes
mm above the top of the bed, and estimate its volume. Pass
Contact time 1 h 1 h
NaCl solution (100 g/L) downflow through the single compo- Flow rate, mL/min-mL sample 0.133 0.133
Regeneration level:
nent sample or the separated components of the mixed bed
lb/ft 20.0 21.2
resin at the approximate rate of 0.133 mL/min/mL of sample
g/L 320 340
for 1 h. Discontinue the flow of NaCl solution. Backwash with
D 2187–94 (2004)
TABLE 2 Requirements for Second Pretreatment
14. Procedure
Anion-Exchange Cation-Exchange
14.1 Weigh three approximately 5-g representative samples
Resins Resins
of material pretreated in accordance with Section 10 to the
Reagent HCl NaOH
nearest 1 mg into previously tared weighing vessels.
Concentration 1+9 40 g/L
14.2 Dry the samples for 18 62hat104 6 2°C.
Volume required 8 sample volumes 4 sample volumes
Contact time 1 h 0.5 h
14.3 Remove the samples from the oven. Cool 30 min in a
Flow rate, mL/min-mL sample 0.133 0.133
desiccator, and reweigh.
Regeneration level:
lb/ft 21.2 10.0
15. Calculation
g/L 340 160
15.1 Calculate the water retention capacity, in percent, as
follows:
waterretained,% 5[~A 2 B!/A] 3100 (1)
of thymol blue indicator solution. Continue rinsing until a 100
mL portion of the effluent remains yellow (pH > 2.5) on the
where:
addition of the indicator. Test the effluent from the cation-
A = amount of wet sample used, g, and
exchange resins in the same manner with two drops of
B = amount of dry sample obtained, g.
tropaeolin-O indicator solution. Continue rinsing until a
3 16. Report
100-mLportion of the effluent remains yellow (pH < 11.0) on
the addition of the indicator. 16.1 Report the percent water retained as the average of the
10.12 Removetheion-exchangeresinfromthepretreatment three values obtained.
column, discarding any extraneous material that may have
17. Precision and Bias
accumulated at the bottom of the bed. Transfer the resin to the
17.1 Precision—The precision of this test method of deter-
Buchner funnel of the draining apparatus that has been fitted
mining water retention capacity of ion exchange resins may be
with a medium porosity filter paper. Drain the water to the top
expressed as follows:
of the sample using suction if required. Cover the funnel with
a suitable vacuum-tight cover, which is fitted with an inlet for
S 50.017x
T
air from the water-filled humidifying tower. Apply sufficient
S 50.004x
o
suction to maintain a pressure differential of 40 65mmHg
below atmospheric pressure. Continue passing humidified air where:
through the sample for 10 min. S = overall precision,
T
10.13 Transfertheentiredrainedsampletoaclean,dry,1-L S = single-operator precision, and
o
x = water retention capacity determined in percent.
(1-qt.), wide-mouthed bottle with a screw top or other vapor-
17.1.1 Information given for the precision statement is
tight closure.
derived from round robin testing in which eight laboratories,
i
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.