ASTM D3266-91(2018)
(Test Method)Standard Test Method for Automated Separation and Collection of Particulate and Acidic Gaseous Fluoride in the Atmosphere (Double Paper Tape Sampler Method)
Standard Test Method for Automated Separation and Collection of Particulate and Acidic Gaseous Fluoride in the Atmosphere (Double Paper Tape Sampler Method)
SIGNIFICANCE AND USE
5.1 This test method provides a means of automatically separating and collecting atmospheric particulate and acidic gaseous fluoride samples.
5.2 Since the samples are collected on dry tapes, the samples are in a form which allows elution of the fluoride content with a small volume of eluent. Consequently, the method allows analyses of air samples taken for a time period as short as several minutes.
SCOPE
1.1 This test method describes the automatic separation and collection on chemically treated paper tapes of particulate and gaseous forms of acidic fluorides in the atmosphere by means of a double paper tape sampler. The sampler may be programmed to collect and store individual air samples obtained over time periods from several minutes to 3 h. A 30.5-m (100-ft) tape will allow unattended operation for the automatic collection of up to 600 samples.
1.2 The values stated in SI units are to be regarded as standard. The values given in parentheses after SI units are included for information only and are not considered standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: D3266 − 91 (Reapproved 2018)
Standard Test Method for
Automated Separation and Collection of Particulate and
Acidic Gaseous Fluoride in the Atmosphere (Double Paper
Tape Sampler Method)
This standard is issued under the fixed designation D3266; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the U.S. Department of Defense.
1. Scope D1356 Terminology Relating to Sampling and Analysis of
Atmospheres
1.1 This test method describes the automatic separation and
D1357 Practice for Planning the Sampling of the Ambient
collection on chemically treated paper tapes of particulate and
Atmosphere
gaseous forms of acidic fluorides in the atmosphere by means
D3195/D3195M Practice for Rotameter Calibration
of a double paper tape sampler. The sampler may be pro-
D3268 Test Method for Separation and Collection of Par-
grammed to collect and store individual air samples obtained
ticulate and Gaseous Fluorides in the Atmosphere (So-
over time periods from several minutes to 3 h. A 30.5-m
dium Bicarbonate-Coated Glass Tube and Particulate
(100-ft) tape will allow unattended operation for the automatic
Filter Method)
collection of up to 600 samples.
D3269 Test Methods for Analysis for Fluoride Content of
1.2 The values stated in SI units are to be regarded as
the Atmosphere and Plant Tissues (Manual Procedures)
standard. The values given in parentheses after SI units are
(Withdrawn 2010)
included for information only and are not considered standard.
D3270 Test Methods for Analysis for Fluoride Content of
1.3 This standard does not purport to address all of the the Atmosphere and Plant Tissues (Semiautomated
safety concerns, if any, associated with its use. It is the
Method)
responsibility of the user of this standard to establish appro- D3609 Practice for Calibration Techniques Using Perme-
priate safety, health, and environmental practices and deter-
ation Tubes
mine the applicability of regulatory limitations prior to use. D3614 Guide for Laboratories Engaged in Sampling and
1.4 This international standard was developed in accor- Analysis of Atmospheres and Emissions
dance with internationally recognized principles on standard-
3. Terminology
ization established in the Decision on Principles for the
Development of International Standards, Guides and Recom-
3.1 Definitions—For definitions of terms used in this test
mendations issued by the World Trade Organization Technical
method, refer to Terminology D1356.
Barriers to Trade (TBT) Committee.
4. Summary of Test Method
2. Referenced Documents
4.1 Air is drawn through an air inlet tube (see Practice
2.1 ASTM Standards: D1357) and is first passed through an acid-treated prefilter
D1071 Test Methods for Volumetric Measurement of Gas- paper tape to remove particulate matter which may contain
eous Fuel Samples fluoride and then through an alkali-treated paper tape to
D1193 Specification for Reagent Water remove acidic fluoride gases.
4.2 The exhaust air is filtered through soda lime-glass wool,
and the cleaned air is used to pressurize the front compartment
This test method is under the jurisdiction of ASTM Committee D22 on Air
to prevent fluoride contamination of the paper tapes from the
Quality and is the direct responsibility of Subcommittee D22.03 on Ambient
ambient air.
Atmospheres and Source Emissions.
Current edition approved June 1, 2018. Published June 2018. Originally
4.3 Automatically, at the end of the preset sampling period,
approved in 1973. Last previous edition approved in 2011 as D3266 – 91 (2011).
the vacuum pump is turned off, the tapes are indexed, and after
DOI: 10.1520/D3266-91R18.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on The last approved version of this historical standard is referenced on
the ASTM website. www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D3266 − 91 (2018)
indexing the vacuum pump is turned on. Indexing results in a
“dead time” of several seconds.
4.4 The paper tapes are removed from the sampler after a
selected period of operation and taken to an analytical work
area where the individual sample spots are cut out, treated to
dissolve the fluoride, and analyzed by potentiometric or pho-
4,5,6
tometric methods.
5. Significance and Use
5.1 This test method provides a means of automatically
separating and collecting atmospheric particulate and acidic
gaseous fluoride samples.
5.2 Since the samples are collected on dry tapes, the
samples are in a form which allows elution of the fluoride
content with a small volume of eluent. Consequently, the
method allows analyses of air samples taken for a time period
as short as several minutes.
6. Interferences
6.1 Particulate metallic salts, such as those of aluminum,
iron, calcium, magnesium or rare-earth elements, may react
with and remove some or all of the acidic gaseous fluoride on
the prefilter. If interfering quantities of such particulate metal-
lic salts are present, the use of Test Method D3268 is
recommended because the acidic fluoride gases are collected
FIG. 1 Dual Tape Sampler Flow Schematic
prior to the filter.
6.2 Acid aerosols or gases might neutralize or acidify the
alkali-treated tape and prevent quantitative uptake of the acidic 7. Apparatus
fluoride gases from the atmosphere. If this potential interfer-
7.1 The double paper tape sampler is a modification of and
ence is present the decreased alkalinity of the water extract
utilizes the basic principles of the sequential paper tape
(13.2.2.1) may provide relevant information.
sampler used for dust collection. The commercially available
apparatus requires modification, as described in this test
6.3 Aluminum or certain other metals or phosphates can
method, prior to use. It consists of the following:
interfere with subsequent analyses of the tapes by photometric
7.1.1 Heated Inlet—I , TFE-fluorocarbon, 1 m (3.3 ft) in
or electrometric methods. These potential interferences are
length,9.5mm( ⁄8in.)(outsidediameter),encasedina9.5mm
discussed in Test Methods D3269 and D3270.
( ⁄8 in.) (inside diameter) aluminum tube. See Fig. 1. The
6.4 There are several limitations of the test method that
aluminum jacket is wrapped in a constant wattage heating wire
could possibly occur:
of 25 W/m (8 W/ft). The tube is connected to the instrument
with a TFE-fluorocarbon fitting.
6.4.1 Although the acid-treated medium retentive prefilter
7.1.1.1 Rainshield, R —Constructed of TFE-fluorocarbon.
has been shown to allow passage of hydrofluoric acid, it will
s
7.1.1.2 Proportional Temperature Controller—H , with
restrict passage of particulate matter only as small as about 1
thermocouple reference point located at the bottom of the
µm.Thus,smallerparticulatemattermaypassthroughthefilter
sample chamber.
and impinge on or pass through the alkali-treated second tape.
7.1.1.3 Inlet Thermostat—T .
6.4.2 The maximum sampling time recommended in the
7.1.1.4 Inlet Pressure Gauge—M with shutoff valve, V .
5 1
method is 3 h. This time is limited to minimize the possible
One side of the gauge is connected to a TFE-fluorocarbon run
effect of particulate matter sorbing the acidic fluoride gases or
tee placed between the intake tube and the sample block, and
reducing the sampling rate.
The sole source of supply of the apparatus known to the committee at this time
Mandl, R. H., Weinstein, L. H., Weiskopf, G. J., and Major, J. L., “The isAnderson Samplers,Atlanta, GA. If you are aware of alternative suppliers, please
Separation and Collection of Gaseous and Particulate Fluorides,” Paper CP-25A, provide this information toASTM International Headquarters. Your comments will
2D International Clean Air Congress, Washington, DC, 1970. receive careful consideration at a meeting of the responsible technical committee,
Weinstein,L.H.,andMandl,R.H.,“TheSeparationandCollectionofGaseous which you may attend.
and Particulate Fluorides,” VDI Berichte Nr., Vol 164, 1971, pp. 53–63. Zankel, K. L., McGirr, R., Romm, M., Campbell, S. A., Miller, R. “Measure-
Lodge, James P. Jr., ed., “Methods ofAir Sampling andAnalysis,” Intersociety ment of Ambient Ground-Level Concentrations of Hydrogen Fluoride,” Journal of
Committee, 3rd ed., Lewis Publishers, Inc., 1988, pp. 352–356. The Air Pollution Control Association, Vol 37, 1987, pp. 1191–1196.
D3266 − 91 (2018)
the other side is connected to a TFE-fluorocarbon run tee lower block shall be lowered by means of an electric solenoid
placed at the entrance to the intake tubing. which counteracts the spring pressure.
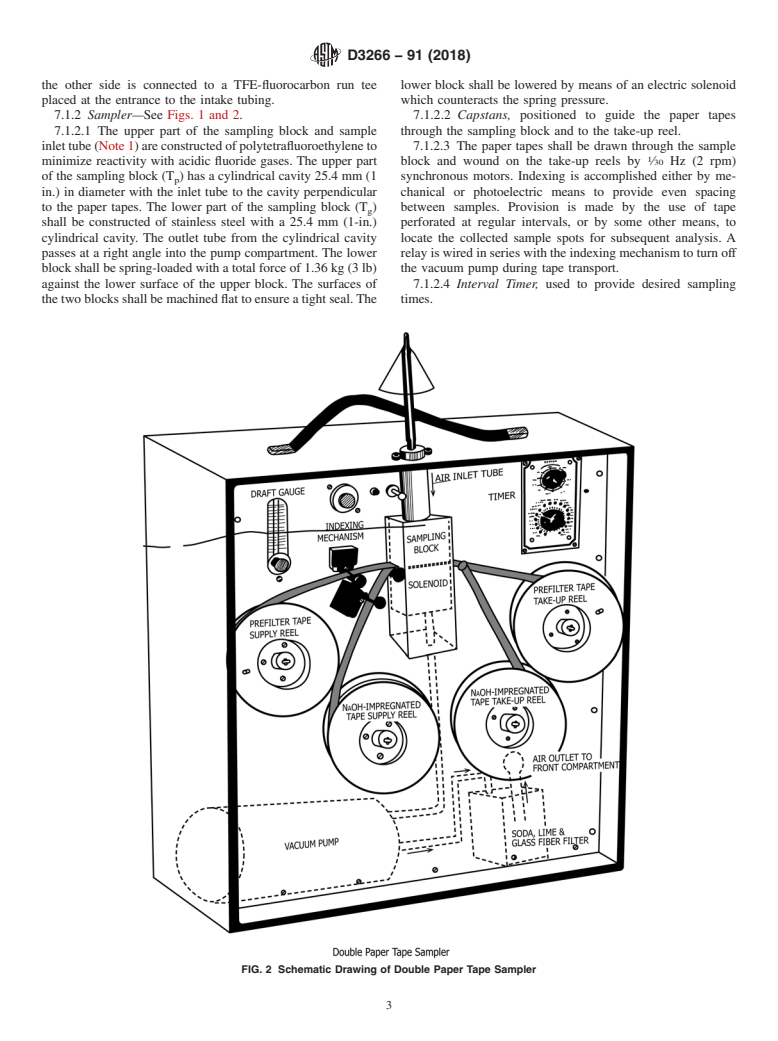
7.1.2 Sampler—See Figs. 1 and 2. 7.1.2.2 Capstans, positioned to guide the paper tapes
7.1.2.1 The upper part of the sampling block and sample through the sampling block and to the take-up reel.
inlettube(Note1)areconstructedofpolytetrafluoroethyleneto 7.1.2.3 The paper tapes shall be drawn through the sample
minimize reactivity with acidic fluoride gases. The upper part block and wound on the take-up reels by ⁄30 Hz (2 rpm)
of the sampling block (T ) has a cylindrical cavity 25.4 mm (1 synchronous motors. Indexing is accomplished either by me-
p
in.) in diameter with the inlet tube to the cavity perpendicular chanical or photoelectric means to provide even spacing
to the paper tapes. The lower part of the sampling block (T ) between samples. Provision is made by the use of tape
g
shall be constructed of stainless steel with a 25.4 mm (1-in.) perforated at regular intervals, or by some other means, to
cylindrical cavity. The outlet tube from the cylindrical cavity locate the collected sample spots for subsequent analysis. A
passes at a right angle into the pump compartment. The lower relay is wired in series with the indexing mechanism to turn off
block shall be spring-loaded with a total force of 1.36 kg (3 lb) the vacuum pump during tape transport.
against the lower surface of the upper block. The surfaces of 7.1.2.4 Interval Timer, used to provide desired sampling
thetwoblocksshallbemachinedflattoensureatightseal.The times.
FIG. 2 Schematic Drawing of Double Paper Tape Sampler
D3266 − 91 (2018)
7.1.2.5 Carbon-Vane Vacuum Pump, to sample air, of nomi- 8. Reagents and Materials
nal 30 L/min (1 ft /min) free-air capacity. This provides a
8.1 Purity of Reagents—All reagents shall conform to the
sampling rate through two tapes of about 15 L/min (0.5
specifications of the Committee on Analytical Reagents of the
ft /min). Exhaust air from the pump is passed through a soda
American Chemical Society, where such specifications are
lime-glass wool filter (S ) and the filtered air is used to 9
p
available.
pressurizethefrontcompartmentandpreventcontaminationby
8.2 Purity of Water—Water shall be Grade II Reagent
fluorides from the ambient air.
conforming to Specification D1193. Additionally, the water
used in the sampling and analytical procedures shall be
demonstrated by testing with a specific ion electrode or by
concentration and photometric analysis to contain less than
0.005 µg/mm of fluoride.
8.3 Chemically treated medium retentive filter paper tape
38-mm (1.5-in.) wide shall be used as the prefilter.
8.4 Chemically treated soft open filter paper 38-mm (1.5-
in.) wide shall be used to remove acidic gaseous fluorides.
8.5 Citric Acid, Alcoholic, Solution (0.1 M)—Dissolve
4.203 g of citric acid monohydrate in 200 mL of 95 % ethyl
alcohol.
8.6 Sodium Hydroxide, Alcoholic Glycerin Solution (0.5
N)—Dissolve4.000gofNaOHpelletsin200mLof95 %ethyl
alcohol containing 5 % glycerol.
8.7 Total Ionic Strength Adjustment Buffer (TISAB)—Add
57 mL of glacial acetic acid, 58 g of NaCl and 4.0 g of CDTA
((1,2-cyclohexylenedinitrilo)tetraacetic acid) to 500 mL of
distilled water. Stir and add 5 N NaOH solution (8.11) slowly
until pH is between 5.0 and 5.5. Cool and dilute to 1 L.
8.8 TISAB (1+1) —Dilute the full strength TISAB (8.7)
FIG. 3 Inlet Flow Calibration Schematic
1 + 1 with an equal amount of reagent water.
8.9 Sulfuric Acid (1.0 N)—Add 28.0 mL of concentrated
H SO (sp gr 1.84) to 250 mL of reagent water in a 1-L
2 4
volumetric flask. Swirl to mix, cool, and dilute to 1 L with
7.1.2.6 Sample Flow Adjustment Valve—An inline needle
reagent water. Mix thoroughly.
valve, V .
8.10 Sodium Hydroxide Solution (1.0 N)—Dissolve 40.0 g
7.1.2.7 Flow Indicator—0–30 L/min (0–1 ft /min) M .
of NaOH in 250 mLof reagent water in a 1000-mLvolumetric
7.1.2.8 Paper Tape—38-mm (1.5-in.) wide, appropriately
flask. Swirl to mix, cool, and dilute to 1000 mL with reagent
treated chemically (10.1).
water. Mix thoroughly.
7.1.2.9 Provision shall be made for manual override of the
tape transport mechanism.
8.11 Sodium Hydroxide Solution(5.0 N)Dissolve 200.0 g of
7.1.2.10 All fittings shall be constructed of TFE-
NaOH in a 1-Lvolumetric flask. Swirl to mix, cool, and dilute
fluorocarbon.
to 1 L with water. Mix thoroughly.
7.2 Calibration Equipment—See Fig. 3.
8.12 Hydrogen Fluoride Permeation Tube—200 ng/min at
7.2.1 Inlet CalibrationAdapter—To connect hose from flow
35°C is satisfactory.
calibration equipment to sampler inlet.
9. Sampling
7.2.2 Flow Meter—M , 0–30 L/min (0–1 ft /min), cali-
brated in accordance with Practice D3195/D3195M.
9.1 See Practice D1357 for general sampling guidelines.
7.2.3 Wet Testmeter—M ,calibratedinaccordancewithTest
Methods D1071.
Reagent Chemicals, American Chemical Society Specifications, American
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
7.3 HF Permeation Tube Calibrator—A permeation tube
listed by the American Chemical Society, see Analar Standards for Laboratory
device, modified as described in Footnote 8. See also Practice
Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
D3609.All components of the calibrator that come into contact
and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
with HF shall be constructed of TFE-fluorocarbon.
MD.
D3266 − 91 (2018)
9.2 Carefully align the sample block assembly to minimize 11.8 Draw a calibratio
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.