ASTM D6703-01
(Test Method)Standard Test Method for Automated Heithaus Titrimetry
Standard Test Method for Automated Heithaus Titrimetry
SCOPE
1.1 This test method describes a procedure for quantifying three Heithaus compatibility parameters that estimate the colloidal stability of asphalts and asphalt cross blends (1,2), aged asphalts (3), and pyrolyzed heavy oil residua and asphalt (4) using automated Heithaus titrimetry as a stability diagnostic tool.
1.2 >This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D 6703 – 01
Standard Test Method for
Automated Heithaus Titrimetry
This standard is issued under the fixed designation D 6703; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope manufactured, composed principally of high-molecular weight
hydrocarbons, of which asphalts, tars, pitches, and asphaltites
1.1 This test method describes a procedure for quantifying
are typical.
three Heithaus compatibility parameters that estimate the
2 2.1.7 coke, n—the solid product resulting from the destruc-
colloidal stability of asphalts and asphalt cross blends (1,2) ,
tive distillation of coal, petroleum residuum, or bitumen in an
aged asphalts (3), and pyrolyzed heavy oil residua and asphalt
ovenorclosedchamber,orfromimperfectcombustionofthese
(4)usingautomatedHeithaustitrimetryasastabilitydiagnostic
materials, consisting principally of carbon.
tool.
2.1.8 colloidal suspension, n—an intimate mixture of two
1.2 This standard does not purport to address all of the
substances, one of which, called the dispersed phase (or
safety concerns, if any, associated with its use. It is the
colloid), is uniformly distributed in a finely divided state
responsibility of the user of this standard to establish appro-
throughthesecondsubstance,calledthedispersionmedium(or
priate safety and health practices and determine the applica-
dispersing medium).
bility of regulatory limitations prior to use.
2.1.9 compatibility, n—the state of peptization of an as-
2. Terminology phalt, which is measured quantitatively by the Heithaus param-
eter P.
2.1 Definitions of Terms Specific to This Standard:
2.1.10 core asphalts, n—the eight asphalts selected for
2.1.1 asphalt (5), n—a dark brown to black cementitious
intensive study in the Strategic Highway Research Program
material, solid or semisolid in consistency, in which the
(SHRP).
predominating constituents are bitumens, which occur in na-
2.1.11 dispersed phase, n—one phase of a dispersion con-
ture as such or are obtained as residue by refining petroleum.
sisting of particles or droplets of one substance distributed
2.1.2 asphalt cross-blend, n—any mixture of two or more
through a second phase.
asphalts blended together to form a consistent material.
2.1.12 dispersingmedium,n—onephaseofadispersionthat
2.1.3 asphaltene peptizability, n—the tendency of asphalt-
distributes particles or droplets of another substance, the
enes to exist as a stable dispersion in a maltene solvent,
disperse phase.
measured by the Heithaus parameter p .
a
2.1.13 flocculation, n—the process of aggregation and coa-
2.1.4 asphaltenes, n—the high molecular weight hydrocar-
lescence into a flocculent mass.
bon fraction precipitated from asphalt by a designated paraf-
2.1.14 Heithaus compatibility parameters, n—three param-
finic naphtha solvent at a specified solvent-asphalt ratio.
eters: asphaltene peptizability (p ), maltene peptizing power
2.1.4.1 Discussion—The asphaltene fraction should be a
(p ), and asphalt state of peptization (P), measured using
identified by the solvent and solvent-asphalt ratio used. o
Heithaus titration methods.
2.1.5 asphalt state of peptization, n—a measure of the
2.1.15 maltene peptizing power, n—the ability of a maltene
ability of the combination of a maltene solvent and dispersed
solvent to disperse asphaltenes, measured by the Heithaus
asphaltenes to form a stable dispersed system. Equivalent to
parameter p .
o
compatibility of the system.
2.1.16 maltenes, n—a red-brown to black heavy oil material
2.1.6 bitumen, n—a class of black or dark-colored (solid,
derived from asphalt after precipitation with normal or
semisolid, or viscous) cementitious substances, natural or
branched alkanes (for example, n-pentane, n-hexane,
n-heptane, isooctane, and so forth), filtration of asphaltenes,
and distillation of alkane precipitating agent from the filtrate.
This test method is under the jurisdiction of ASTM Committee D04 on Road
and Paving Materials and is the direct responsibility of Subcommittee D04.47 on
Equivalent to deasphaltened materials. Maltenes are the sol-
Miscellaneous Asphalt Tests.
vent moiety of an asphalt.
Current edition approved Aug. 10, 2001. Published October 2001.
2 2.1.17 oxidatively age-hardened asphalt, n—an asphalt that
The boldface numbers in parentheses refer to the list of references at the end of
this standard. has reacted with oxygen at elevated temperatures in an oven,
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D 6703
usually under greater than atmospheric oxygen pressure. The 3.3 Thespectrophotometeroutputsignaldetectstheonsetof
reaction is run for a time sufficient to simulate asphalt aging in turbidity of the sample solution. This is the flocculation onset
pavement. point, corresponding to the beginning of the precipitation of
2.1.18 pyrolysis, n—the breaking apart of complex mol- asphaltenesfromthesamplesolution.Fig.3illustratesatypical
ecules into simpler units by the use of heat, as in the pyrolysis seriesofplotsof%Tversus tforthethreetestsolutions.Values
of heavy oil to make gasoline. of %T increase with time until maximum values of %T are
2.1.19 residuum, n—a quantity or body of matter remaining observed, after which values of %T decrease. The reason that
after evaporation, combustion, or distillation. the curves in Fig. 3 exhibit maxima is that, at the beginning of
each titration, %T increases due to dilution with titrant. At the
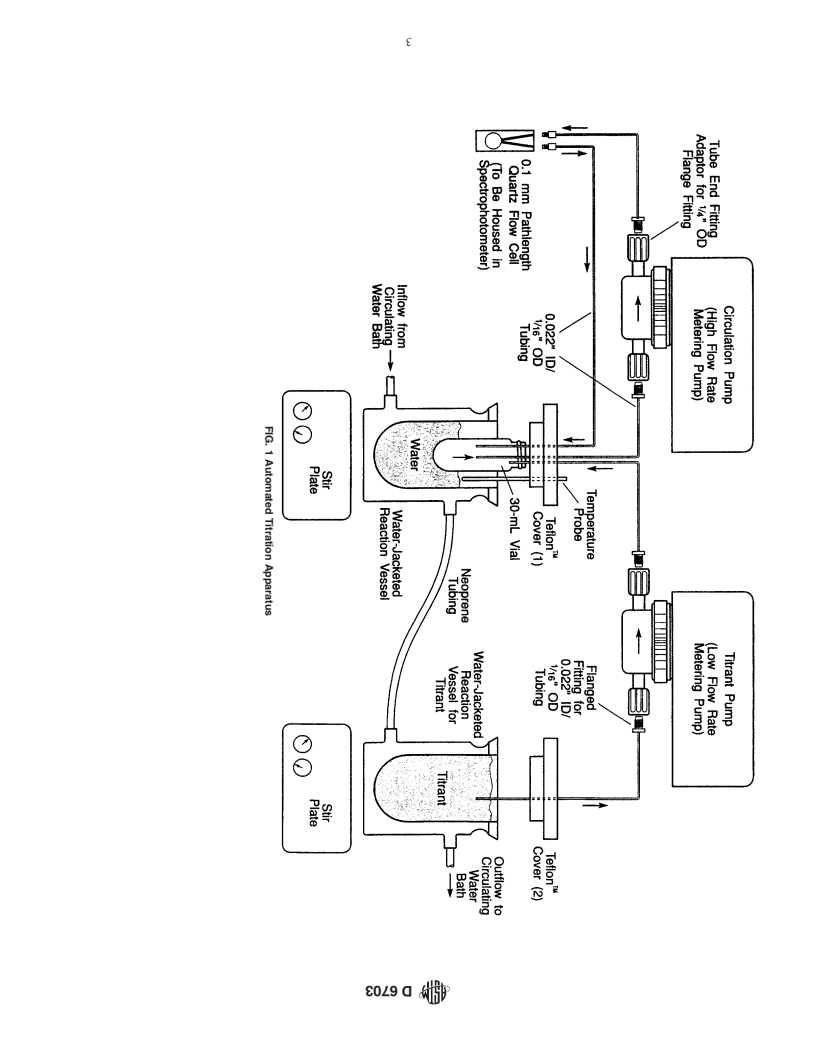
3. Summary of Test Method
flocculation onset point, the formation of asphaltene particles
3.1 Three 30-mL reaction vials are tared. Three samples of
causes an immediate decrease in %T due to light scattering
an asphalt (or a heavy oil residuum), one weighing 0.400 g, a
effects. The time required to reach the maximum in %T from
second weighing 0.600 g, and the third weighing 0.800 g, are
the onset of titration of a sample is defined as the flocculation
transferred to the reaction vials, one sample into each vial.
time, t. When the value of t for each sample is multiplied by
f f
Toluene (2.000 mL) is added to each of the reaction vials to
the titrant flow rate, the titrant volume, V , required to cause
T
dissolve the asphalt (or heavy oil residuum). Thus, each
the onset of flocculation for each sample is obtained.
reaction vial contains a solution of different concentration of
3.4 The weight of each asphalt (or heavy oil residuum)
asphalt (or heavy oil residuum) in toluene. Each of these
sample, W , the volume of toluene used to dissolve each
a
solutions then is titrated with isooctane (2,2,4-trimethyl pen-
sample, V (2.00 mL in each case), and the volume of titrant
S
tane) or some other titrant (6) at a constant titrant delivery rate.
required to cause the onset of flocculation, V are recorded for
T
The titration is performed by installing the reaction vials
each sample solution. Values of these three quantities for each
separately in the apparatus illustrated in Fig. 1. Basically, this
set of three test sample solutions are used to calculate the
apparatus consists of intersecting sample circulation and titra-
quantities C (referred to as the dilution concentration) and FR
tion loops.
(referred to as the flocculation ratio). C is defined as W /(V +
a S
3.2 Each reaction vial is housed in a 200-mL, water-
V ). FR is defined as V /(V + V ). Values of C are plotted
T S S T
jacketed reaction vessel (Fig. 1). Water-jacketing is required
versus FR for each of the three recorded sets of values of W ,
a
because careful temperature control of the system is essential.
V , and V (Fig. 4). Customarily, the C values are along the
S T
The reaction vessel is filled with enough water such that the
x-axis, and the FR values are the y-axis. The three data points
reaction vial and temperature probe are immersed. Water
are connected by a line, and the line is extrapolated to both
flowing through the water jacket maintains the temperature of
axes.Thepointatwhichthelineinterceptsthe x-axisisdefined
the water in the reaction vessel, which maintains the tempera-
as C . The point at which the line intercepts the y-axis is
min
tureofthesolutioninthereactionvial.Theconnectionwiththe
defined as FR . These two values are used to calculate the
max
sample circulation loop is made by covering the reaction vial
three Heithaus compatibility parameters, designated p , p , and
a o
with a screw top TFE-fluorocarbon cover penetrated by three
P.The parameter p , the peptizability of asphaltenes, is defined
a
small bore TFE-fluorocarbon tubes (Fig. 2). A fourth hole in
as the quantity (1 – FR ). The parameter p , the peptizing
max o
the cover accommodates a temperature probe. One of these
power of maltenes, is defined as the quantity FR [(1/C )+
max min
tubes (1.6-mm ( ⁄16-in. diameter)) leads to a short path length
1]. The parameter P, the overall compatibility of the system, is
(0.1-mm) quartz cell housed in a ultraviolet- (UV) visible
defined as [p /(1 – p )], or (1/C + 1).
o a min
spectrophotometer. A second tube (1.6-mm diameter) leads to
4. Significance and Use
ahighflowratemeteringpumpandthentothequartzcell.This
system is the sample circulation loop. The third tube (1.6-mm 4.1 This test method is intended primarily as a laboratory
diameter) connects the reaction vial to the titration loop and diagnostic tool for estimating the colloidal stability of asphalt,
leads to a low flow rate metering pump and then to another asphalt cross blends, aged asphalt, pyrolyzed asphalt, and
water-jacketed reaction vessel filled with titrant (usually iso- heavy oil residuum. Historically, asphalt and heavy oil residua
octane). This reaction vessel is covered with another TFE- have been modeled as colloidal suspensions (8,9) in which a
fluorocarbon cover penetrated by one TFE-fluorocarbon tube. polar, associated asphaltene moiety (the dispersed phase) is
This TFE-fluorocarbon cover has the same dimensions as the suspended in a maltene solvent moiety (the dispersing me-
one illustrated in Fig. 2, but does not require threading because dium). The extent to which these two moieties remain in a
the cover fits directly over the reaction vessel and no vial is given state of peptization is a measure of the compatibility of
screwed into it.Also it has only one hole. The second reaction the suspension. Compatibility influences important physical
vesselisfilledwithtitrant.Whilethesamplesolutioncirculates properties of these materials, including rheological properties,
through the sample circulation loop, the titrant is pumped into for example, phase angle and viscosity (10,11). Compatibility
the sample reaction vial at a constant rate using the low flow also influences coke formation in refining processes (4). This
ratemeteringpump.Duringthisprocess,theoutputsignalfrom test method and other similar test methods (7, 12-15), along
the spectrophotometer is recorded using an integrator or some with the classical Heithaus test (1,2), measures the overall
other data gathering device. The change in percent transmit- compatibilityofacolloidalsystembydeterminingadesignated
tance (%T) of detected radiation at 740 nm (7) passing through parameter referred to as the state of peptization, P. The value
the quartz cell is plotted versus the time, t, during which the of P commonly varies between 2.5 and 10 for unmodified or
titrant is added to the sample reaction vial. neat asphalts. Materials calculated to have low values of P are
D 6703
FIG. 1 Automated Titration Apparatus
D 6703
FIG. 2 Reaction Vial (30 mL) with TFE-fluorocarbon Cover and Temperature Probe
designated as incompatible, where as materials calculated to residuum or asphalt (W ), the volume of solvent (V ), and the
a S
have high P values are designated as compatible. Values of P volume of titrant added up to the flocculation point (V ).
T
may be calculated as a function of two other designated
5. Apparatus
parameters that relate to the peptizability of the asphaltene
moiety (the asphaltene peptizability parameter, p ) and the 5.1 UV-visible Spectrophotometer, wavelength scanning
a
solvent power of the maltene moiety (the maltene peptizing range from 200 to 1000 nm, with adjustable aperture or
power parameter, p ). Values of p and p are calculated as attenuator.
o a o
functions of the quantities C and FR , the values of which
5.2 Digital Integrator, or data acquisition system (com-
min max
are obtained from three experimental variables, the weight of puter). One-millisecond data sampling rate.
D 6703
FIG. 3 Onset of Flocculation Peaks Measured at Three Successively Increasing Concentrations (Solvent: Toluene, Titrant: Isooctane)
FIG. 4 Flocculation Ratio Versus Dilution Concentration for One Stable Asphalt and One Less Stable Asphalt
5.3 Water-Jacketed Reaction Vessel, 200-mL, two. 5.5 High Flow Rate Metering Pump—Piston diameter, 3.0
mm ( ⁄8 in.); piston displacement < 0.1 mL; flow rate range
5.4 TFE-fluorocarbon Covers, two.
from1.0to20.0mL/min;flowrateconsistency, 60.1mL/min;
5.4.1 TFE-fluorocarbon Cover No. 1, (see Fig. 2), threaded
and piston chamber resistant to damage from solvent contact.
to hold a 30-mL reaction vial. Dimensions: thickness, 2.0 mm
5.6 Low Flow Rate Metering Pump—Piston diameter, 3.0
9 3
( ⁄16in.); diameter, 70 mm (2 ⁄4 in.), threaded to 30-mLreaction
mm ( ⁄8 in.); flow rate range from 0.100 to 1.000 mL/min; flow
vial. Three holes, 1.5 mm ( ⁄16 in.) in diameter, concentric to
rateconsistency, 60.001mL/min;andpistonchamberresistant
the cover’s center, are arranged in a triangle, are tapped to set
to damage from solvent contact.
within the inside diameter of the vial when attached to the
5.7 Magnetic Stirring Plates, two.
TFE-fluorocarbon cover, with a distance between holes
5.8 Refrigerated Water Bath Circulator (for greater tem-
roughly equal to 10 mm ( ⁄8 in.). One additional hole, 3.0 mm
1 perature control)—Temperature control in this procedure is at
( ⁄8 in.), is tapped off center, positioned just to the outside of
25°C (77°F). Temperature variation, 60.1°C (0.2°F); tempera-
where the reaction vial is positioned in the TFE-fluorocarbon
ture range from 0 to 100°C (32 to 212°F). Coupled to fit
cover. This hole allows the temperature probe to be inserted
neoprene tubing, 13-mm ( ⁄2-in.) in inside diameter.
into the water-filled reaction vessel.
5.9 Quartz Flow Cell, 0.1-mm path length with tube end
5.4.2 TFE-fluorocarbon Cover No. 2,
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.