ASTM D301-95(2004)
(Test Method)Standard Test Methods for Soluble Cellulose Nitrate (Withdrawn 2011)
Standard Test Methods for Soluble Cellulose Nitrate (Withdrawn 2011)
SIGNIFICANCE AND USE
Ash accounts for the nonsoluble, nonfilm forming portion of the polymer. It may affect solution clarity and film properties.
SCOPE
1.1 These test methods cover the material known as soluble cellulose nitrate (also known as soluble nitrocellulose), which is shipped wet in conformance with regulations of the Interstate Commerce Commission.
1.2 The test methods appear in the following sections: SectionsAshDrying SamplesNitrogenStabilityToluene DilutionViscosity
1.3 The values stated in SI units are to be regarded as the standard. Values given in parentheses are for information only.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For specific hazard statements, see 12.2, 13.3, 16.1, and 16.2.
WITHDRAWN RATIONALE
These test methods cover the material known as soluble cellulose nitrate (also known as soluble nitrocellulose), which is shipped wet in conformance with regulations of the Interstate Commerce Commission.
Formerly under the jurisdiction of Committee D01 on Paint and Related Coatings, Materials, and Applications, this test method was withdrawn in June 2011. This standard is being withdrawn without replacement because of its limited use by industry.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Federation of Societies for
Designation:D301–95(Reapproved2004) Paint Technology Standard No. Cs-2-58
Standard Test Methods for
Soluble Cellulose Nitrate
This standard is issued under the fixed designation D301; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
This standard has been approved for use by agencies of the Department of Defense.
1. Scope E1 Specification for ASTM Liquid-in-Glass Thermometers
1.1 These test methods cover the material known as soluble
3. Sampling
cellulose nitrate (also known as soluble nitrocellulose), which
3.1 Samples shall be taken from not less than 10 % (at least
isshippedwetinconformancewithregulationsoftheInterstate
two barrels) of each lot or batch in the shipment. In sampling
Commerce Commission.
the barrels, two samples of approximately 0.5 dm (1 pt) each
1.2 The test methods appear in the following sections:
shall be taken from two well-separated points at least 0.3 m (1
Sections
ft) beneath the surface of the material in the barrel. These
Ash 5-7
samples shall then be composited to represent each lot or batch
Drying Samples 4
in the shipment.
Nitrogen 8-10
3.2 The samples shall meet the following requirements:
Stability 11-13
Toluene Dilution 22-24
3.2.1 Appearance—Thecellulosenitrateshallnotbediscol-
Viscosity 14-18
ored and shall be free of lumps and foreign matter, such as
1.3 The values stated in SI units are to be regarded as the
charred particles.
standard. Values given in parentheses are for information only. 3.2.2 Ash—Ash content shall not exceed 0.30 %, calculated
1.4 This standard does not purport to address all of the
on the basis of dry-weight soluble cellulose nitrate.
safety concerns, if any, associated with its use. It is the 3.2.3 Nitrogen—The percent nitrogen, calculated on the
responsibility of the user of this standard to establish appro-
basisofdry-weightsolublecellulosenitrate,shallbewithinthe
priate safety and health practices and determine the applica- limits agreed upon by the purchaser and the manufacturer for
bility of regulatory limitations prior to use. For specific hazard
the particular type of soluble cellulose nitrate.
statements, see 12.2, 13.3, 16.1, and 16.2. 3.2.4 Stability—The stability as determined by the 134.5 C
test shall be not less than 25 min.
2. Referenced Documents
3.2.5 Viscosity—The viscosity shall be within the limits
2.1 ASTM Standards: agreed upon by the purchaser and the manufacturer for the
D302 Specification for Ethyl Acetate (85 to 88 Percent
particular type of soluble cellulose nitrate.
Grade) 3.2.6 Solubility and Appearance of the Solution—The solu-
D303 Specification forn-ButylAcetate (90 to 92 % Grade)
bility and appearance of the sample shall be equal to the
D362 Specification for Industrial Grade Toluene reference standard for the particular type of soluble cellulose
D1343 Test Method for Viscosity of Cellulose Derivatives
nitrate.
by Ball-Drop Method 3.2.7 Film Test—The film test of the sample shall be equal
D4795 Test Method for Nitrogen Content of Soluble
to that of the reference standard for the particular type of
Nitrocellulose—Alternative Method soluble cellulose nitrate.
3.2.8 Toluene Dilution Test—The toluene dilution value of
the sample shall be equivalent to that of the reference standard
These test methods are under the jurisdiction of ASTM Committee D01 on
for the particular type of soluble cellulose nitrate.
Paint and Related Coatings, Materials, and Applications and are the direct
responsibility of Subcommittee D01.36 on Cellulose and Cellulose Derivatives.
DRYING SAMPLES
Current edition approved June 1, 2004. Published June 2004. Originally
approved in 1929. Last previous edition approved in 1999 as D301 – 95 (1999).
DOI: 10.1520/D0301-95R04.
4. Procedure
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
4.1 Soluble cellulose nitrate is a flammable material, the
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
degree of flammability varying with the extent and nature of
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website.
the wetting medium. Cellulose nitrate is always wet with water
Withdrawn. The last approved version of this historical standard is referenced
or alcohol in commercial handling, shipping, and storage, in
on www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D301–95 (2004)
which condition it presents no unusual hazard. Dry cellulose 9. Calculation
nitrate, if ignited by fire, spark, or static electricity, burns very
9.1 Calculate the percent ash as follows:
rapidly. Samples of dry cellulose nitrate must not be stored at
Ash, %5~wt of ash/wt of dry sample!3 100
any time. Dry only that portion required for immediate test.
Wet the excess material and the samples left after testing with
10. Precision and Bias
water and dispose of by burning on a safe burning ground.
10.1 Precision—Statistical analysis of intralaboratory (re-
4.2 Dry small quantities required for ash and nitrogen tests
peatability) test results on a sample containing approximately
by spreading in a thin layer on a tray at room temperature for
0.015 % ash indicates a precision of 60.015 % absolute at the
12 to 16 h, followed by oven-drying in crucibles or weighing
95 % confidence level.
bottles1hat100to 105°C. The oven used for drying cellulose
10.2 Bias—No statement of bias can be made as no suitable
nitrate should have the latch removed. Wear a face mask (see
reference material is available as a standard.
12.2) when the oven is opened after samples have been heated.
4.3 Dry larger quantities of water-wet material required for
NITROGEN
viscosity and toluene dilution tests, or a small quantity for
stability tests, by blowing warm compressed air (at a tempera-
11. Significance and Use
ture of 60 to 65°C, and a pressure of 275 to 415 kPa (40 to 60
11.1 The nature and strength of solvent systems required for
psi)) through the sample placed in a cylindrical holder with a
cellulose nitrate are dependent upon the nitrogen content.
screen over one end for ⁄2 to 1 h. Provide the compressed air
Mismatches of solvent with nitrogen level can result in poor
line with a safety plug (Note 1) of Wood’s metal, which melts
solution quality and colloid and gel formation.
at 70 to 75°C, so the air will be diverted from the sample if a
11.2 An alternative preferred method can be found in Test
temperature of 70°C is exceeded.
Method D4795.
NOTE 1—Information on the availability of a suitable fusible plug
assembly may be obtained from ASTM International Headquarters.
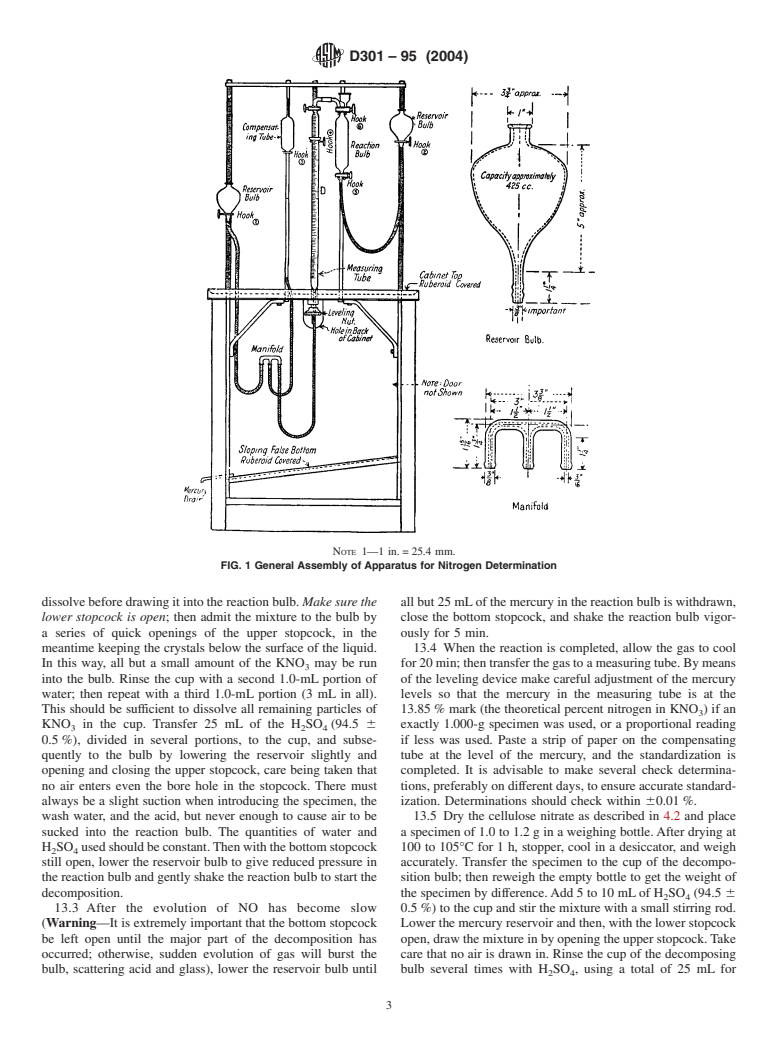
12. Apparatus
4.4 If the material is alcohol-wet, it is necessary to modify
12.1 Nitrometer—Use the duPont Nitrometer, which is il-
the drying procedure. After placing the required amount of
lustrated in Figs. 1-4.
cellulose nitrate in the cylindrical holder, pour in sufficient
12.2 Face Mask—Aface mask, so constructed that a heavy
distilled or iron-free water to fill it.Allow the bulk of the liquid
piece of cellulose acetate sheeting protects the face.
to drain off. Then dry by blowing warm air through the holder
(Warning—The cellulose acetate mask must be worn during
as described in 4.3.
the generation and measurement of the gas as a precaution in
case of an explosion.)
ASH
13. Procedure
5. Significance and Use
13.1 Calibrate the measuring tube accurately in the usual
5.1 Ash accounts for the nonsoluble, nonfilm forming por-
manner, using mercury as the calibrating liquid.
tion of the polymer. It may affect solution clarity and film
13.2 Standardize the apparatus as follows:
properties.
13.2.1 Fill the compensating, measuring, and reaction tubes
and their rubber connections with mercury. Run 20 to 30 mLof
6. Apparatus
H SO (ACS grade, 94.5 6 0.5 %) into the reaction bulb
2 4
6.1 Porcelain Crucibles, Coors No. 3 or equivalent.
throughthecupatthetopandadmitabout210mLofair.Close
6.2 Muffle Furnace, maintained at 550 6 25°C.
the stopcocks, shake the bulb well, and allow to stand
overnight. This desiccates the air which is then run into the
7. Reagents
compensating tube until the mercury is about on a level with
7.1 Ethyl Alcohol.
the 12.50 % mark on the measuring tube, the two tubes being
7.2 Acetone.
held at the same height.Then seal the compensating tube using
7.3 Castor Oil.
a small blowpipe flame.
13.2.2 As a preferred alternative, nitrogen may be used in
8. Procedure
place of air.
8.1 Dry the cellulose nitrate as described in 4.2 and place a 13.2.3 Place in weighing bottles 0.95 6 0.05-g portions of
specimen of approximately 4.0 g in a tared and ignited ACS grade KNO that has been recrystallized twice from
crucible. Moisten the sample in the crucible with ethyl alcohol, distilled water and ground to pass a No. 100 (150-µm) sieve.
thengelatinizebyaddingasufficientamountof5 %solutionof Drythespecimens2to3hat135to150°C.Stopperthebottles,
castor oil into the acetone. Place the crucible in a draft-free cool in a desiccator, and weigh accurately. Transfer the KNO
hood and ignite the contents with a Bunsen flame. Allow the to the cup of the reaction bulb and weigh the weighing bottle
material to burn without further addition of heat until a charred to obtain the weight of sample used.Add 1.0 mL of water and
residue remains. Place the crucible in a muffle furnace at 550 stir the mixture in the cup with a small glass stirring rod to
6 25°C for 90 min. Remove carefully the crucible from the liberate the entrained bubbles of air; work the undissolved
muffle furnace to avoid loss of ash, cool in a desiccator, and crystals into the lower part of the cup, keeping them below the
weigh accurately. surface of the solution. It is not necessary that the KNO
D301–95 (2004)
NOTE 1—1 in. = 25.4 mm.
FIG. 1 General Assembly of Apparatus for Nitrogen Determination
dissolve before drawing it into the reaction bulb.Makesurethe all but 25 mLof the mercury in the reaction bulb is withdrawn,
lower stopcock is open; then admit the mixture to the bulb by close the bottom stopcock, and shake the reaction bulb vigor-
a series of quick openings of the upper stopcock, in the ously for 5 min.
meantime keeping the crystals below the surface of the liquid. 13.4 When the reaction is completed, allow the gas to cool
In this way, all but a small amount of the KNO may be run for20min;thentransferthegastoameasuringtube.Bymeans
into the bulb. Rinse the cup with a second 1.0-mL portion of of the leveling device make careful adjustment of the mercury
water; then repeat with a third 1.0-mL portion (3 mL in all). levels so that the mercury in the measuring tube is at the
This should be sufficient to dissolve all remaining particles of 13.85 % mark (the theoretical percent nitrogen in KNO)ifan
KNO in the cup. Transfer 25 mL of the H SO (94.5 6 exactly 1.000-g specimen was used, or a proportional reading
3 2 4
0.5 %), divided in several portions, to the cup, and subse- if less was used. Paste a strip of paper on the compensating
quently to the bulb by lowering the reservoir slightly and tube at the level of the mercury, and the standardization is
opening and closing the upper stopcock, care being taken that completed. It is advisable to make several check determina-
no air enters even the bore hole in the stopcock. There must tions, preferably on different days, to ensure accurate standard-
always be a slight suction when introducing the specimen, the ization. Determinations should check within 60.01 %.
wash water, and the acid, but never enough to cause air to be 13.5 Dry the cellulose nitrate as described in 4.2 and place
sucked into the reaction bulb. The quantities of water and a specimen of 1.0 to 1.2 g in a weighing bottle.After drying at
H SO usedshouldbeconstant.Thenwiththebottomstopcock 100 to 105°C for 1 h, stopper, cool in a desiccator, and weigh
2 4
still open, lower the reservoir bulb to give reduced pressure in accurately. Transfer the specimen to the cup of the decompo-
the reaction bulb and gently shake the reaction bulb to start the sition bulb; then reweigh the empty bottle to get the weight of
decomposition. the specimen by difference.Add 5 to 10 mLof H SO (94.5 6
2 4
13.3 After the evolution of NO has become slow 0.5 %) to the cup and stir the mixture with a small stirring rod.
(Warning—It is extremely important that the bottom stopcock Lower the mercury reservoir and then, with the lower stopcock
be left open until the major part of the decomposition has open, draw the mixture in by opening the upper stopcock.Take
occurred; otherwise, sudden evolution of gas will burst the care that no air is drawn in. Rinse the cup of the decomposing
bulb, scattering acid and glass), lower the reservoir bulb until bulb several times with H SO , using a total of 25 mL for
2 4
D301–95 (2004)
NOTE 1—1 in. = 25.4 mm.
FIG. 3 Reaction Bulb
made to hold 13 to 15 test tubes.To aid in heat transfer, add 15
NOTE 1—1 in. = 25.4 mm.
to 25 mLof mineral oil to each copper well, in order to fill the
FIG. 2 Measuring Tube for Nitrogen Determination
spacebetweentheglasstubeandthewell.Tomaintainthebath
at a temperature of 134.5 6 0.5°C, fill to within 3 in. (76 mm)
of the top with a mixture consisting of ten parts of a
dissolving and rinsing. Complete the determination in accor-
commercial ethylene glycol solution (automobile radiator an-
dance with the procedure described in 10.2 for standardization
tifreeze containing a corrosion inhibitor) and one part of water.
of the apparatus, and take a reading after adjusting the level of
the mercury in the reading tube to the mark on the compen- Adjustthetemperatureoftheboilingliquidinthebathto134.5
6 0.5°C by adding more glycol or water, as necessary.
sating tube.The reading divided by the weight of the specimen
gives the percent nitrogen. 15.2 Test Tubes—Heat-resistant glass tubes, with an out-
side diameter of 18 mm, a wall thickness of 1.5 mm, and a
STABILITY
length of 290 mm.
15.3 Heater—An electric hot plate for heating the bath.
14. Significance and Use
15.4 Face Mask—See 12.2.
14.1 Nitrocellulose stability is measured by detecting the
15.5 Gloves—A pair of heavy gloves.
evolution of nitrogen oxides under elevated temperature. The 15.6 Pincers—Long pincers for handling the test tubes.
results are not necessarily a predictor of shelf life.
15.7 Thermometer—An ASTM Stability Test Thermometer
having a range from 130 to 140°C and
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.