ASTM D4503-86(2003)
(Practice)Standard Practice for Dissolution of Solid Waste by Lithium Metaborate Fusion
Standard Practice for Dissolution of Solid Waste by Lithium Metaborate Fusion
SIGNIFICANCE AND USE
A knowledge of the inorganic constituent composition in a waste is often required for the selection of appropriate waste disposal practices. Solid waste may exist in a variety of forms and contain a range of organic and inorganic constituents. This practice describes a drying and ashing step that may be applied to remove moisture and volatile and nonvolatile organic constituents prior to determining nonvolatile metals. Generation of a dry ash concentrates the inorganic constituents of interest and makes the LiBO2 fusion feasible for a greater variety of waste samples. Acidification of the LiBO2 fusion mix results in a solution amenable to inductively coupled plasma (ICP) or atomic absorption spectrometry (AAS) analysis.
SCOPE
1.1 This practice covers the drying, ashing, and solubilization of solid waste using a lithium metaborate (LiBO2) fusion for the subsequent determination of inorganic constituents by argon plasma emission spectroscopy or atomic absorption spectroscopy.
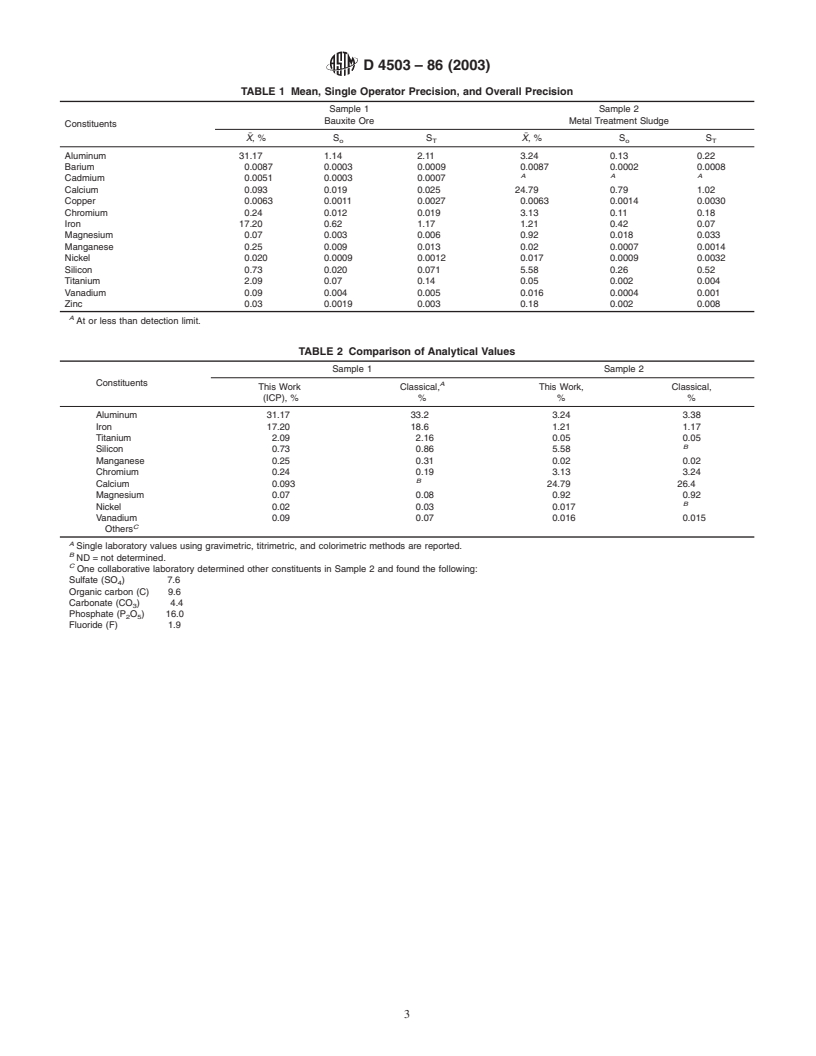
1.2 The following elements may be solubilized by this practice:aluminumchromiumsiliconbariumirontitaniumcadmiummagnesiumvanadiumcalciummanganesezinccoppernickel
1.3 This practice has been used successfully with a bauxite ore and a neutralized metal treatment sludge. The practice may be applicable to other elements not listed above. Some metals, such as cadmium and zinc, may volatilize from some samples during the drying, ashing, or fusion steps. The analyst is responsible for determining whether the practice is applicable to the solid waste being tested.
1.4 This practice is intended for the solubilization of nonvolatile inorganic constituents in solid waste. The LiBO2 fusion is appropriate for a silicate matrix or acid resistant samples.
1.5 This standard does not purport to address all of the safety problems associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. For specific hazard statements see Section 7.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D4503–86 (Reapproved 2003)
Standard Practice for
Dissolution of Solid Waste by Lithium Metaborate Fusion
This standard is issued under the fixed designation D 4503; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope D 3682 Test Method for Major and Minor Elements in Coal
and Coke Ash by Atomic Absorption
1.1 This practice covers the drying, ashing, and solubiliza-
E50 Practices for Apparatus, Reagents, and Safety Precau-
tion of solid waste using a lithium metaborate (LiBO ) fusion
tions for Chemical Analysis of Metals
for the subsequent determination of inorganic constituents by
argon plasma emission spectroscopy or atomic absorption
3. Summary of Practice
spectroscopy.
3.1 Thesolidwasteisweighed,dried,andashedat550°Cto
1.2 The following elements may be solubilized by this
remove water and organic constituents, and reweighed. A
practice:
known portion of the ground ash is mixed with LiBO in a
aluminum chromium silicon
graphite crucible and fused at 1000°C. Immediately after
barium iron titanium
cadmium magnesium vanadium
fusion, the molten mass is poured directly into stirred dilute
calcium manganese zinc
HNO solution, dissolved, filtered, and made to appropriate
copper nickel
volume for subsequent analysis.
1.3 This practice has been used successfully with a bauxite
ore and a neutralized metal treatment sludge. The practice may 4. Significance and Use
be applicable to other elements not listed above. Some metals,
4.1 A knowledge of the inorganic constituent composition
such as cadmium and zinc, may volatilize from some samples
in a waste is often required for the selection of appropriate
during the drying, ashing, or fusion steps. The analyst is
waste disposal practices. Solid waste may exist in a variety of
responsible for determining whether the practice is applicable
forms and contain a range of organic and inorganic constitu-
to the solid waste being tested.
ents. This practice describes a drying and ashing step that may
1.4 This practice is intended for the solubilization of non-
be applied to remove moisture and volatile and nonvolatile
volatile inorganic constituents in solid waste. The LiBO
2 organic constituents prior to determining nonvolatile metals.
fusion is appropriate for a silicate matrix or acid resistant
Generation of a dry ash concentrates the inorganic constituents
samples.
of interest and makes the LiBO fusion feasible for a greater
1.5 This standard does not purport to address all of the
variety of waste samples. Acidification of the LiBO fusion
safety problems associated with its use. It is the responsibility
mix results in a solution amenable to inductively coupled
of the user of this standard to establish appropriate safety and
plasma (ICP) or atomic absorption spectrometry (AAS) analy-
health practices and determine the applicability of regulatory
sis.
limitations prior to use. For specific hazard statements see
5. Apparatus
Section 7.
5.1 Analytical Balance, sensitive to 0.1 mg.
2. Referenced Documents
5.2 Fusion Muffle Furnace, electrically heated, capable of
2.1 ASTM Standards:
maintaining a temperature of 1000°C.
D 1193 Specification for Reagent Water
5.3 Ashing Muffle Furnace, electrically heated, capable of
D 2777 Practice for Determination of Precision and Bias of
maintaining a temperature of 550°C6 30°C and with an
Applicable Methods of Committee D-19 on Water
adequate air circulation. This may be accomplished by con-
necting rubber tubing to a controlled source of clean dry air.
Then, via a ceramic tube inserted into a convenient muffle
This practice is under the jurisdiction of ASTM Committee D34 on Waste
opening, flow approximately 4 L/min of air into the furnace.
Management and is the direct responsibility of Subcommittee D34.01.06 on
5.4 Drying Oven, capable of operating at a temperature up
Analytical Methods.
Current edition approved Nov. 28, 1986. Published February 1987.
to 150°C.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
5.5 Evaporating/Ashing Dish, 50 to 100-mL capacity, made
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
of platinum, silica, or porcelain.
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D4503–86 (2003)
5.6 Fusion Crucibles, graphite, 28 to 30-mL capacity. 8.4 Place the sample into an ashing furnace set at about
5.7 Stirring Hot Plate, capable of operating at a surface 300°C and increase heat gradually so the furnace reaches
temperature up to 300°C with TFE-fluorocarbon-coated stir 550°C in 1 h. Ash at 550°C until no carbonaceous matter is
magnet. apparent. Stirring the sample once an hour may increase the
5.8 Mortar and Pestle, agate or mullite type. oxidation of carbonaceous matter. The ashing time required
5.9 Sieve and Pan,ASTM U.S. Standard Testing Sieve, 200 will depend on the nature of the sample. Several hours, or even
m (75 µm opening). overnight, may be required by difficult-to-ash samples.
5.10 Desiccator.
8.5 Remove the ashing dish and sample from the muffle,
cool in a desiccator, and weigh to determine the combined loss
6. Reagents and Materials
on ashing and drying.
6.1 Purity of Reagents—Reagent grade chemicals shall be
8.6 Quantitatively transfer the ash to a mortar and grind to
used in all tests. Unless otherwise indicated, it is intendedthat
pass a No. 200 sieve, if necessary. Transfer back to the ashing
all reagents shall conform to the specifications of the Commit-
dish and reheat the ground ash at 550°C for 1 h, remove from
tee onAnalytical Reagents of theAmerican Chemical Society,
the ashing furnace and cool in a desiccator. Transfer quantita-
where such specifications are available. Other grades may be
tively to a weighing bottle. Weigh approximately 0.3 g of
used provided it is first ascertained that the reagent is of
sample to the nearest 0.0001 g by difference into a graphite
sufficiently high purity to permit its use without lessening the
crucible containing 1.5 g of LiBO . Mix the ash and LiBO
2 2
accuracy of the determination.
well, then add an additional 0.5 g of LiBO on top of the mix.
6.2 PurityofWater—Unless otherwise indicated, references
NOTE 1—Ashing at 550°C typically gives a free flowing or friable ash,
to water shall be understood to mean Type
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.