ASTM F2694-16(2020)
(Practice)Standard Practice for Functional and Wear Evaluation of Motion-Preserving Lumbar Total Facet Prostheses
Standard Practice for Functional and Wear Evaluation of Motion-Preserving Lumbar Total Facet Prostheses
SIGNIFICANCE AND USE
5.1 Total Facet Prosthesis Components—The total facet replacement may comprise a variety of shapes and configurations. Its forms may include, but are not limited to: ball-and-socket articulating joints, joints having a free-floating or semi-constrained third body, metallic load-bearing surfaces, and spring and dampening mechanisms. Additionally, it may have a unilateral or bilateral design.
5.2 Spinal Testing Apparatus:
5.2.1 Test Chambers—In case of a multispecimen machine, each chamber shall be isolated to prevent cross-contamination of the test specimens. The chamber shall be made entirely of corrosion-resistant materials, such as acrylic plastic or stainless steel, and shall be removable from the machine for thorough cleaning between tests.
5.2.2 Component Clamping/Fixturing—Since the purpose of the test is to characterize the wear and kinematic function of the total facet prosthesis, the method for mounting components in the test chamber shall not compromise the accuracy of assessment of the weight loss or stiffness variation during the test. For example, prostheses having complicated superior and inferior surfaces for contacting bone (for example, sintered beads, hydroxylapatite (HA) coating, plasma spray) may be specially manufactured to modify that surface in a manner that does not affect the wear simulation.
5.2.3 The device should be securely (rigidly) attached at its bone-implant interface to the mating test fixtures.
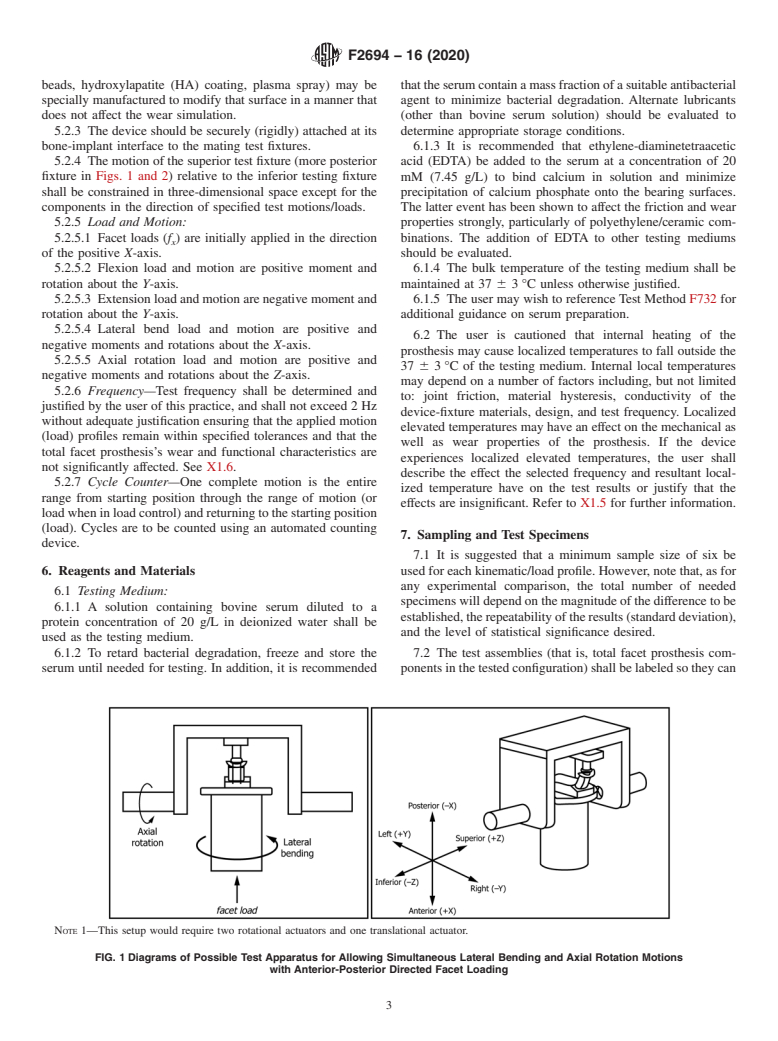
5.2.4 The motion of the superior test fixture (more posterior fixture in Figs. 1 and 2) relative to the inferior testing fixture shall be constrained in three-dimensional space except for the components in the direction of specified test motions/loads.
FIG. 1 Diagrams of Possible Test Apparatus for Allowing Simultaneous Lateral Bending and Axial Rotation Motions with Anterior-Posterior Directed Facet Loading
Note 1: This setup would require two rotational actuators and one translational actuator.
FIG. 2 Diagrams of Po...
SCOPE
1.1 This practice provides guidance for the functional, kinematic, and wear testing of motion-preserving total facet prostheses for the lumbar spine. These implants are intended to allow motion and lend support to the functional spinal unit(s) through replacement of the natural facets.
1.2 This practice is not intended to address the bone implant interface or the static characteristics of the prosthesis components. Fatigue characteristics are included, but only as a by-product of cyclic wear testing under facet load and thus are not addressed in the typical process of generating a Stress-Life (S-N) characterization.
1.3 Biocompatibility of the materials used in a total facet prosthesis are not addressed in this practice.
1.4 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4.1 The values stated in SI units are to be regarded as the standard with the exception of angular measurements, which may be reported in either degrees or radians.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F2694 − 16 (Reapproved 2020)
Standard Practice for
Functional and Wear Evaluation of Motion-Preserving
Lumbar Total Facet Prostheses
This standard is issued under the fixed designation F2694; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
1.1 This practice provides guidance for the functional, 2.1 ASTM Standards:
kinematic, and wear testing of motion-preserving total facet F561 Practice for Retrieval and Analysis of Medical
prostheses for the lumbar spine.These implants are intended to Devices, and Associated Tissues and Fluids
allow motion and lend support to the functional spinal unit(s) F732 Test Method for Wear Testing of Polymeric Materials
through replacement of the natural facets. Used in Total Joint Prostheses
F1714 GuideforGravimetricWearAssessmentofProsthetic
1.2 Thispracticeisnotintendedtoaddresstheboneimplant
Hip Designs in Simulator Devices
interface or the static characteristics of the prosthesis compo-
F1877 Practice for Characterization of Particles
nents. Fatigue characteristics are included, but only as a
F2346 Test Methods for Static and Dynamic Characteriza-
by-product of cyclic wear testing under facet load and thus are
tion of Spinal Artificial Discs
not addressed in the typical process of generating a Stress-Life
F2423 Guide for Functional, Kinematic, and Wear Assess-
(S-N) characterization.
ment of Total Disc Prostheses
1.3 Biocompatibility of the materials used in a total facet
prosthesis are not addressed in this practice. 3. Terminology
1.4 The values stated in SI units are to be regarded as 3.1 All functional and kinematic testing terminology is
standard. No other units of measurement are included in this consistent with the referenced standards, unless otherwise
standard. stated.
1.4.1 The values stated in SI units are to be regarded as the
3.2 Definitions of Terms:
standard with the exception of angular measurements, which
3.2.1 mechanical failure, n—failure associated with a defect
may be reported in either degrees or radians.
in the material (for example, fatigue crack) or the bonding
1.5 This standard does not purport to address all of the
between materials that may or may not produce functional
safety concerns, if any, associated with its use. It is the
failure. [F2423]
responsibility of the user of this standard to establish appro-
3.2.2 runout (cycles), n—maximum number of cycles that a
priate safety, health, and environmental practices and deter-
test needs to be carried to if functional failure has not yet
mine the applicability of regulatory limitations prior to use.
occurred. [F2423]
1.6 This international standard was developed in accor-
3.3 Definitions of Terms Specific to This Standard:
dance with internationally recognized principles on standard-
3.3.1 coordinate systems/axes, n—global XYZ orthogonal
ization established in the Decision on Principles for the
axes are defined following a right-handed Cartesian coordinate
Development of International Standards, Guides and Recom-
system in which the XY plane is parallel to and co-planar with
mendations issued by the World Trade Organization Technical
the superior endplate of the inferior vertebral body. The global
Barriers to Trade (TBT) Committee.
axes are fixed relative to the inferior vertebral body, which in
this practice is also considered to be stationary with respect to
ThispracticeisunderthejurisdictionofASTMCommitteeF04onMedicaland
Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.25 on Spinal Devices. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved Oct. 1, 2020. Published November 2020. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
approved in 2007. Last previous edition approved in 2016 as F2694 – 16. DOI: Standards volume information, refer to the standard’s Document Summary page on
10.1520/F2694-16R20. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
F2694 − 16 (2020)
thetestmachine’sframe.Lowercaseletters, xyz,denotealocal 3.3.13 net volumetric wear NV of wear specimen (mm ),
i
moving orthogonal coordinate system attached to the superior n—NV = NW/ρ at end of cycle interval i; where ρ = mass
i i
vertebralbodywithdirectionsinitiallycoincidentwiththoseof density (for example, units of g/mm ) of the wear material.
the global XYZ axes, respectively. The 3D motion of the
3.3.14 wear, n—progressive loss of material from the de-
superiorrelativetotheinferiorvertebraisspecifiedandistobe
vice(s) or device components as a result of relative motion at
measured in terms of sequential Eulerian angular rotations
the surface with another body as measured by the change in
about the xyz axes, respectively (z axial rotation, x lateral bend,
mass of the total facet prosthesis or components of the total
and y flexion-extension). See Figs. 1 and 2.
facet prosthesis. In the case of a non-articulating, compliant
total facet prosthesis, wear is defined simply as the loss of
3.3.1.1 origin, n—center of the global coordinate system
that is located at the posterior medial position on the superior material from the prosthesis. Note that inferior and superior
bone interface components are excluded from this definition
endplate of the inferior vertebral body.
(see 5.2.2).
3.3.1.2 X-axis, n—the positive X-axis is directed anteriorly
3.3.15 facet load, n—anterior-posterior (AP) directed force
relative to the specimen’s initial unloaded position. See Figs. 1
(applied in the direction of the global X-axis) representing the
and 2.
resultant in the mid-sagittal XZ plane applied by the superior
3.3.1.3 Y-axis, n—the positive Y-axis is directed laterally
vertebra that simulates the in-vivoAPshear load F transmitted
x
(toward the left) relative to the specimen’s initial unloaded
from superior to inferior vertebra and resisted by the total facet
position. See Figs. 1 and 2.
prosthesis.
3.3.1.4 Z-axis, n—the positive Z-axis is directed superiorly
relative to the specimen’s initial unloaded position. See Figs. 1
4. Summary of Practice
and 2.
4.1 This practice can be used to describe the function,
3.3.2 fluid absorption, n—fluid absorbed by the device
kinematics, and wear behavior of total facet prostheses sub-
material during testing or while implanted in vivo.
jected to cyclic loading/motion for relatively large numbers of
cycles. (For example, various designs of total facet prostheses,
3.3.3 functional failure, n—permanent deformation or wear
as well as the effects of materials, manufacturing techniques
that renders the total facet prosthesis assembly ineffective or
and other design variables on one particular design can be
unable to perform its intended function.
studied using this practice.)
3.3.4 interval net volumetric wear rate, VR, during cycle
i
interval i (mm /million cycles), n—VR = WR/ρ; where ρ = 4.2 This practice is intended to be applicable to total facet
i i
prostheses that support and transmit motion by means of an
mass density (for example, units of g/mm ) of the wear
material. articulating joint or by use of compliant materials. Ceramics,
metals, and/or polymers may be used in total facet prosthesis
3.3.5 interval net wear rate, WR, during cycle interval i
i
design, and it is the goal of this practice to enable a kinematic
(g/million cycles), n—WR =((NW – NW )/(number of cycles
i i i-1
wear comparison of these devices, regardless of material and
in interval i))·106; for i=1, NW =0.
i-1
type of device.
3.3.6 total facet prosthesis, n—nonbiologic structure in-
tended to restore the support and motion of the natural
5. Significance and Use
vertebral facet joint.
5.1 Total Facet Prosthesis Components—The total facet
3.3.7 kinematics profile, n—relative motion between adja-
replacement may comprise a variety of shapes and configura-
cent vertebral bodies that the total facet prosthesis is subjected
tions. Its forms may include, but are not limited to: ball-and-
to while being tested.
socket articulating joints, joints having a free-floating or
semi-constrained third body, metallic load-bearing surfaces,
3.3.8 load profile, n—loading that the device experiences
while being tested under a defined kinematics profile or the and spring and dampening mechanisms. Additionally, it may
have a unilateral or bilateral design.
loading that the total facet prosthesis is subject to if tested in
load control.
5.2 Spinal Testing Apparatus:
5.2.1 Test Chambers—In case of a multispecimen machine,
3.3.9 radius of rotation, n—the distance between the center
of rotation and the functional position (for example, load- each chamber shall be isolated to prevent cross-contamination
bearing contact point) of the total facet prosthesis, for a given of the test specimens. The chamber shall be made entirely of
motion (that is, flexion/extension, lateral bending, or axial corrosion-resistantmaterials,suchasacrylicplasticorstainless
rotation). steel, and shall be removable from the machine for thorough
cleaning between tests.
3.3.10 weight S of soak control specimen (g), n—S initial
i 0
5.2.2 Component Clamping/Fixturing—Since the purpose
and S at end of cycle interval i.
i
of the test is to characterize the wear and kinematic function of
3.3.11 weight W of wear specimen (g), n—W initial and W
i 0 i
the total facet prosthesis, the method for mounting components
at end of cycle interval i.
in the test chamber shall not compromise the accuracy of
3.3.12 net wear NW of wear specimen (g), n—NW =(W – assessment of the weight loss or stiffness variation during the
i i 0
W)+(S – S ); loss in weight of the wear specimen corrected test. For example, prostheses having complicated superior and
i i 0
for fluid absorption at end of cycle interval i. inferior surfaces for contacting bone (for example, sintered
F2694 − 16 (2020)
beads, hydroxylapatite (HA) coating, plasma spray) may be thattheserumcontainamassfractionofasuitableantibacterial
specially manufactured to modify that surface in a manner that agent to minimize bacterial degradation. Alternate lubricants
does not affect the wear simulation. (other than bovine serum solution) should be evaluated to
5.2.3 The device should be securely (rigidly) attached at its determine appropriate storage conditions.
bone-implant interface to the mating test fixtures. 6.1.3 It is recommended that ethylene-diaminetetraacetic
5.2.4 The motion of the superior test fixture (more posterior acid (EDTA) be added to the serum at a concentration of 20
fixture in Figs. 1 and 2) relative to the inferior testing fixture mM (7.45 g/L) to bind calcium in solution and minimize
shall be constrained in three-dimensional space except for the precipitation of calcium phosphate onto the bearing surfaces.
components in the direction of specified test motions/loads. The latter event has been shown to affect the friction and wear
5.2.5 Load and Motion: properties strongly, particularly of polyethylene/ceramic com-
5.2.5.1 Facet loads (f ) are initially applied in the direction binations. The addition of EDTA to other testing mediums
x
of the positive X-axis. should be evaluated.
5.2.5.2 Flexion load and motion are positive moment and 6.1.4 The bulk temperature of the testing medium shall be
rotation about the Y-axis. maintained at 37 6 3 °C unless otherwise justified.
5.2.5.3 Extensionloadandmotionarenegativemomentand 6.1.5 The user may wish to reference Test Method F732 for
rotation about the Y-axis.
additional guidance on serum preparation.
5.2.5.4 Lateral bend load and motion are positive and
6.2 The user is cautioned that internal heating of the
negative moments and rotations about the X-axis.
prosthesis may cause localized temperatures to fall outside the
5.2.5.5 Axial rotation load and motion are positive and
37 6 3 °C of the testing medium. Internal local temperatures
negative moments and rotations about the Z-axis.
may depend on a number of factors including, but not limited
5.2.6 Frequency—Test frequency shall be determined and
to: joint friction, material hysteresis, conductivity of the
justified by the user of this practice, and shall not exceed 2 Hz
device-fixture materials, design, and test frequency. Localized
without adequate justification ensuring that the applied motion
elevated temperatures may have an effect on the mechanical as
(load) profiles remain within specified tolerances and that the
well as wear properties of the prosthesis. If the device
total facet prosthesis’s wear and functional characteristics are
experiences localized elevated temperatures, the user shall
not significantly affected. See X1.6.
describe the effect the selected frequency and resultant local-
5.2.7 Cycle Counter—One complete motion is the entire
ized temperature have on the test results or justify that the
range from starting position through the range of motion (or
effects are insignificant. Refer to X1.5 for further information.
loadwheninloadcontrol)andreturningtothestartingposition
(load). Cycles are to be counted using an automated counting
7. Sampling and Test Specimens
device.
7.1 It is suggested that a minimum sample size of six be
6. Reagents and Materials used for each kinematic/load profile. However, note that, as for
any experimental comparison, the total number of needed
6.1 Testing Medium:
specimenswilldependonthemagnitudeofthedifferencetobe
6.1.1 A solution containing bovine serum diluted to a
established,therepeatabilityoftheresults(standarddeviation),
protein concentration of 20 g/L in deionized water shall be
and the level of statistical significance desired.
used as the testing medium.
6.1.2 To retard bacterial degradation, freeze and store the 7.2 The test assemblies (that is, total facet prosthesis com-
serum until needed for testing. In addition, it is recommended ponentsinthetestedconfiguration)shallbelabeledsotheycan
NOTE 1—This setup would require two rotational actuators and one translational actuator.
FIG. 1 Diagrams of Possible Test Apparatus for Allowing Simultaneous Lateral Bending and Axial Rotation Motions
with Anterior-Posterior Directed Facet Loading
F2694 − 16 (2020)
NOTE 1—This setup would require two rotational actuators and one translational actuator.
FIG. 2
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.