ASTM E1010-84(2004)
(Practice)Standard Practice for Preparation of Disk Specimens of Steel and Iron for Spectrochemical Analysis by Remelting
Standard Practice for Preparation of Disk Specimens of Steel and Iron for Spectrochemical Analysis by Remelting
SIGNIFICANCE AND USE
Most spectrochemical instruments employed for analyzing steel and iron require a solid specimen with a flat surface large enough for analytical excitation and measurement procedures. This practice describes a procedure for converting unusual types of steel and iron samples to satisfactory spectrochemical specimens.
SCOPE
1.1 This practice describes the preparation of disk specimens of steel and iron by melting chunks, chips, drillings, turnings, wire, or powder briquets with an electric arc in an argon atmosphere. Solidification of the specimen takes place in the crucible in an argon atmosphere. The disk obtained is suitable for quantitative spectrochemical analysis.
1.2 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices, and determine the applicability of regulatory limitations prior to use. Specific precautionary statements are given in 6.2.1, and Section 8.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:E1010–84(Reapproved2004)
Standard Practice for
Preparation of Disk Specimens of Steel and Iron for
Spectrochemical Analysis by Remelting
This standard is issued under the fixed designation E1010; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope crucible in an argon atmosphere. After solidification, the
specimen is removed from the crucible and prepared for
1.1 This practice describes the preparation of disk speci-
spectrochemical analysis.
mens of steel and iron by melting chunks, chips, drillings,
4.2 Partial losses of some elements may be experienced
turnings, wire, or powder briquets with an electric arc in an
during the preparation of the disk specimen. This procedure, if
argon atmosphere. Solidification of the specimen takes place in
carefully followed, will provide consistent losses. Elemental
the crucible in an argon atmosphere. The disk obtained is
losses can be determined by correlating the analysis of the
suitable for quantitative spectrochemical analysis.
charge material with the spectrochemical analysis of the
1.2 This standard does not purport to address all of the
remelted specimen.
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
5. Significance and Use
priate safety and health practices, and determine the applica-
5.1 Most spectrochemical instruments employed for analyz-
bility of regulatory limitations prior to use. Specific precau-
ing steel and iron require a solid specimen with a flat surface
tionary statements are given in 6.2.1, and Section 8.
large enough for analytical excitation and measurement proce-
2. Referenced Documents dures. This practice describes a procedure for converting
unusual types of steel and iron samples to satisfactory spec-
2.1 ASTM Standards:
trochemical specimens.
E135 Terminology Relating to Analytical Chemistry for
Metals, Ores, and Related Materials
6. Apparatus
E876 Practice for Use of Statistics in the Evaluation of
3 6.1 Melting Furnace, consisting of a chamber that
Spectrometric Data
contains the following:
3. Terminology 6.1.1 Crucible, of copper and water-cooled, in which
samples of steel or iron are melted, then solidified to form
3.1 For definitions of terms used in this procedure, refer to
specimens for spectrochemical analysis.
Terminology E135.
6.1.2 Electrode Holder, water-cooled and of negative polar-
4. Summary of Practice
ity, that can be moved up and down easily, and may have
provisions for circular motion and adjusting the arc gap to a
4.1 The sample of steel or iron is placed in a water-cooled
fixed spacing.
copper crucible. The furnace is flushed with argon at a
6.1.3 Viewing Window, compared of dark welding-type
controlled rate of flow. An arc is struck between the electrode
glass with an inner-protective glass that is impervious to heat
and the sample material and is maintained until the melting is
and splatter from the molten metal.
complete. The molten specimen is allowed to solidify in the
6.2 D-C Electric Power Generator, to supply electric cur-
rent and voltage equivalent to that required for electric arc
This practice is under the jurisdiction of ASTM Committee E01 on Analytical
welding. It may be a rotating d-c generator or a static rectifier
Chemistry for Metals, Ores and Related Materials and is the direct responsibility of
with provisions to adjust the current in the 0 to 600 A range.
Subcommittee E01.01 on Iron, Steel, and Ferroalloys.
6.2.1 Caution—A safety interlock shall be provided to
Current edition approved Oct. 1, 2004. Published November 2004. Originally
approved in 1984. Last previous edition approved in 2000 as E1010 – 84 (2000).
prevent electrical shocks to the operator when the melting
DOI: 10.1520/E1010-84R04.
furnace is open.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website.
3 4
Withdrawn. The last approved version of this historical standard is referenced Melting furnaces, manufactured by Hankison Corp., Cannonsburg, PA 15317
on www.astm.org. and Zeebac Inc., Berea, OH 44017, have been found suitable for this purpose.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
E1010–84 (2004)
6.3 Vacuum Pump, with free air capacity of 50 L/min and This prevents loss of sample due to splattering of the powder
vacuum of 350 µm, minimum. when the arc is first struck.
NOTE 1—Ifthedeterminationofcarboninthespecimenisrequired,use
7. Materials
a thoriated-tungsten electrode. If the determination of tungsten or thorium
7.1 Inert Gas, argon of 99.96 % purity.
is required, use a graphite electrode.
7.2 Electrode, thoriated tungsten or high-purity graphite.
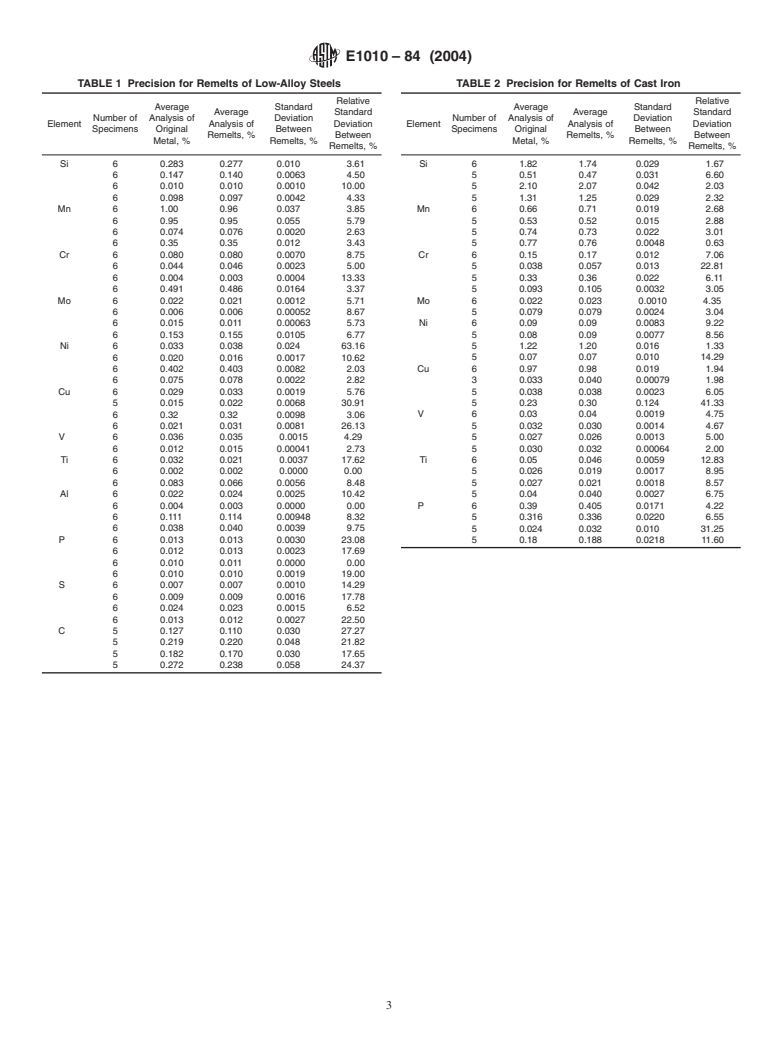
11. Precision and Bias
8. Safety Precautions
11.1 Precision:
8.1 Operating personnel should adhere to the manufactur-
11.1.1 Tables 1-3 show the percent standard deviations and
er’s operating recommendations to avoid electrical shock and
the percent relative standard deviations among disks of various
physical harm due to light and heat. See 6.2.1 and 10.1.2.1 for
melted ferrous metals analyzed with both optical emission
specific precautions.
spectrometers and X-ray fluorescence spectrometers. The pre-
cision data are included to serve as a guide for the precision
9. Preparation of Samples
obtainablefrommeltedspecimenspreparedasdescribedinthis
9.1 Remove grease from samples and dry before melting.
practice. The data were calculated in accordance with Practice
Remove other surface contaminates by suitable methods. For
E876.
consistent melting, fine powders, chips, drillings, turnings, or
11.1.2 Therelativestandarddeviationsamongmeltedspeci-
wire may be compacted in a briquetting press with 35-mm die
mens can be quite large. The large deviations are due to
at a pressure of 2800 kgf/mm .
element losses or enrichment during melting which can be
minimized by good melting technique, particularly for carbon,
10. Preparation of Specimens
sulfur, and copper. Cleaning the crucible between melts can
10.1 Place 40 to 50 g of sample in the crucible. Close the
reduce contamination errors, especially when widely differing
furnace. The melting of the sample and solidification of the
materials are melted. The physical appearance of the melted
specimen may vary slightly depending on the design of the
specimenswillsometimesbeanindicationofthehomogeneity.
furnace and the type of metal being prepared. Two suggested
11.2 Bias:
procedures are as follows:
11.2.1 The data in Tables 1-3 show the average analyses for
10.1.1 Procedure A—The following steps are programmed
ferrous metals before melting and for melted specimens.While
automaticallyafterpressingthestartbutton:(1)Flushingofthe
the majority of the average analyses of melted specimens
crucib
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.