ASTM E685-93(2005)
(Practice)Standard Practice for Testing Fixed-Wavelength Photometric Detectors Used in Liquid Chromatography
Standard Practice for Testing Fixed-Wavelength Photometric Detectors Used in Liquid Chromatography
SIGNIFICANCE AND USE
Although it is possible to observe and measure each of the several characteristics of a detector under different and unique conditions, it is the intent of this practice that a complete set of detector specifications should be obtained under the same operating conditions. It should also be noted that to completely specify a detector’capability, its performance should be measured at several sets of conditions within the useful range of the detector. The terms and tests described in this practice are sufficiently general that they may be used regardless of the ultimate operating parameters.
Linearity and response time of the recorder or other readout device used should be such that they do not distort or otherwise interfere with the performance of the detector. This requires adjusting the gain, damping, and calibration in accordance with the manufacturer’directions. If additional electronic filters or amplifiers are used between the detector and the final readout device, their characteristics should also first be established.
SCOPE
1.1 This practice is intended to serve as a guide for the testing of the performance of a photometric detector (PD) used as the detection component of a liquid-chromatographic (LC) system operating at one or more fixed wavelengths in the range 210 to 800 nm. Measurements are made at 254 nm, if possible, and are optional at other wavelengths.
1.2 This practice is intended to describe the performance of the detector both independently of the chromatographic system (static conditions) and with flowing solvent (dynamic conditions).
1.3 For general liquid chromatographic procedures, consult Refs (1-9).
1.4 For general information concerning the principles, construction, operation, and evaluation of liquid-chromatography detectors, see Refs (10 and 11) in addition to the sections devoted to detectors in Refs (1-7).
1.5 The values stated in SI units are to be regarded as standard.
1.6 This standard does not purport to address all of the safety problems, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E685 − 93 (Reapproved2005)
Standard Practice for
Testing Fixed-Wavelength Photometric Detectors Used in
Liquid Chromatography
This standard is issued under the fixed designation E685; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope Ultraviolet and Visible Spectrophotometers
E682Practice for Liquid Chromatography Terms and Rela-
1.1 This practice is intended to serve as a guide for the
tionships
testingoftheperformanceofaphotometricdetector(PD)used
as the detection component of a liquid-chromatographic (LC)
3. Terminology
systemoperatingatoneormorefixedwavelengthsintherange
210to800nm.Measurementsaremadeat254nm,ifpossible,
3.1 Definitions:
and are optional at other wavelengths.
3.1.1 absorbance calibration, n—the procedure that verifies
that the absorbance scale is correct within 65%.
1.2 This practice is intended to describe the performance of
thedetectorbothindependentlyofthechromatographicsystem
3.1.2 drift, n—the average slope of the noise envelope
(static conditions) and with flowing solvent (dynamic condi-
expressed in absorbance units per hour (AU/h) as measured
tions).
over a period of 1 h.
1.3 For general liquid chromatographic procedures, consult
3.1.3 dynamic, n—under conditions of a flow rate of 1.0
Refs (1-9).
mL/min.
1.4 For general information concerning the principles,
3.1.4 linear range, n— of a PD, the range of concentrations
construction, operation, and evaluation of liquid-
of a test substance in a mobile phase over which the response
chromatography detectors, see Refs (10 and 11) in addition to
ofthedetectorisconstanttowithin5%asdeterminedfromthe
the sections devoted to detectors in Refs (1-7).
linearity plot specified below and illustrated in Fig. 1. The
linear range should be expressed as the ratio of the highest
1.5 The values stated in SI units are to be regarded as
concentration to the minimum detectable concentration or the
standard. No other units of measurement are included in this
lowest linear concentration, whichever is greatest.
standard.
1.6 This standard does not purport to address all of the 3.1.5 long-term noise, n—the maximum amplitude in AU
for all random variations of the detector signal of frequencies
safety problems, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro- between6and60cyclesperhour(0.1and1.0cyclespermin).
priate safety and health practices and determine the applica-
3.1.5.1 Discussion—Itrepresentsnoisethatcanbemistaken
bility of regulatory limitations prior to use.
for a late-eluting peak. This noise corresponds to the observed
noise only and may not always be present.
2. Referenced Documents
3.1.6 minimum detectability, n—of a PD, that concentration
2.1 ASTM Standards:
ofaspecificsoluteinaspecificsolventthatresultsinadetector
E275PracticeforDescribingandMeasuringPerformanceof
response corresponding to twice the static short-term noise.
3.1.7 response time (speed of output), n—the detector, the
timerequiredforthedetectoroutputtochangefrom10to90%
This practice is under the jurisdiction ofASTM Committee E13 on Molecular
of the new equilibrium value when the composition of the
Spectroscopy and Separation Science and is the direct responsibility of Subcom-
mittee E13.19 on Separation Science. mobilephaseischangedinastepwisemanner,withinthelinear
Current edition approved Sept. 1, 2005. Published September 2005. Originally
range of the detector.
approved in 1979. Last previous edition approved in 2000 as E685–93(2000).
3.1.7.1 Discussion—Because the detector volume is very
DOI: 10.1520/E0685-93R05.
small and the transport rate is not diffusion dependent, the
Theboldfacenumbersinparenthesesrefertothelistofreferencesattheendof
this practice.
response time is generally fast enough to be unimportant. It is
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
generally comparable to the response time of the recorder and
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
dependent on the response time of the detector electrometer
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. and on the recorder amplifier. Factors that affect the observed
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E685 − 93 (2005)
5. Noise and Drift
5.1 Test Conditions—Pure, degassed methanol of suitable
grade shallbeusedinthesamplecell.Airornitrogenshallbe
used in the reference cell if there is one. Nitrogen is preferred
where the presence of high-voltage equipment makes it likely
that there is ozone in the air. Protect the entire system from
temperature fluctuations because these will lead to detectable
drift.
5.1.1 The detector should be located at the test site and
turned on at least 24 h before the start of testing. Insufficient
warm-upmayresultindriftinexcessoftheactualvalueforthe
detector.
5.2 Methods of Measurement:
5.2.1 Connect a suitable device (Note 1) between the pump
and the detector to provide at least 75 kPa (500 psi) back
pressure at 1.0 mL/min flow of methanol. Connect a short
length(about100mm)of0.25-mm(0.01-in.)internal-diameter
stainless steel tubing to the outlet tube of the detector to retard
bubble formation. Connect the recorder to the proper detector
FIG. 1 Example of a Linearity Plot for a Photometric Detector output channels.
NOTE 1—Suggested devices include (a)2to4mof 0.1-mm (0.004-in.)
internal-diameter stainless steel tubing, (b) about 250 mm of 0.25 to
0.5-mm (0.01 to 0.02-in.) internal-diameter stainless steel tubing crimped
response time include the true detector response time, elec-
with pliers or cutters, or (c) a constant back-pressure valve located
tronic filtering, and system band-broadening.
between the pump and the injector.
3.1.8 short-term noise, n—the maximum amplitude, peak to
5.2.2 Repeatedly rinse the reservoir and chromatographic
peak, inAU for all random variations of the detector signal of
system, including the detector, with degassed methanol to
a frequency greater than one cycle per minute.
remove from the system all other solvents, any soluble
3.1.8.1 Discussion—Itdeterminesthesmallestsignaldetect-
material, and any entrained gasses. Fill the reservoir with
able by a PD, limits the precision attainable in quantitation of
methanolandpumpthissolventthroughthesystemforatleast
trace-level samples, and sets the lower limit on linearity. This
30 min to complete the system cleanup.
noise corresponds to the observed noise only.
5.2.3 Air or nitrogen is used in the reference cell, if any.
3.1.9 static, n—under conditions of no flow.
Ensure that the cell is clean, free of dust, and completely dry.
5.2.4 To perform the static test, cease pumping and allow
4. Significance and Use
thechromatographicsystemtostabilizeforatleast1hatroom
4.1 Although it is possible to observe and measure each of
temperature without flow. Set the attenuator at maximum
the several characteristics of a detector under different and sensitivity (lowest attenuation), that is, the setting for the
unique conditions, it is the intent of this practice that a
smallest value of absorbance units full-scale (AUFS). Adjust
complete set of detector specifications should be obtained the response time as close as possible to 2 s for a PD that has
under the same operating conditions. It should also be noted
a variable response time (Note 2). Record the response time
that to completely specify a detector’s capability, its perfor- used.Adjustthedetectoroutputtonearmidscaleonthereadout
mance should be measured at several sets of conditions within
device. Record at least1hof detector signal under these
the useful range of the detector. The terms and tests described conditions, during which time the ambient temperature should
in this practice are sufficiently general that they may be used
not change by more than 2°C.
regardless of the ultimate operating parameters.
NOTE2—Timeconstantisconvertedtoresponsetimebymultiplyingby
4.2 Linearity and response time of the recorder or other the factor 2.2. The effect of electronic filtering on observed noise may be
studied by repeating the noise measurements for a series of response-time
readout device used should be such that they do not distort or
settings.
otherwise interfere with the performance of the detector. This
5.2.5 Draw pairs of parallel lines, each pair corresponding
requires adjusting the gain, damping, and calibration in accor-
to between 0.5 and 1 min in length, to form an envelope of all
dance with the manufacturer’s directions. If additional elec-
tronicfiltersoramplifiersareusedbetweenthedetectorandthe
final readout device, their characteristics should also first be 4
Distilled-in-glass or liquid-chromatography grade. Complete freedom from
established. particles may require filtration, for example, through a 0.45-µm membrane filter.
E685 − 93 (2005)
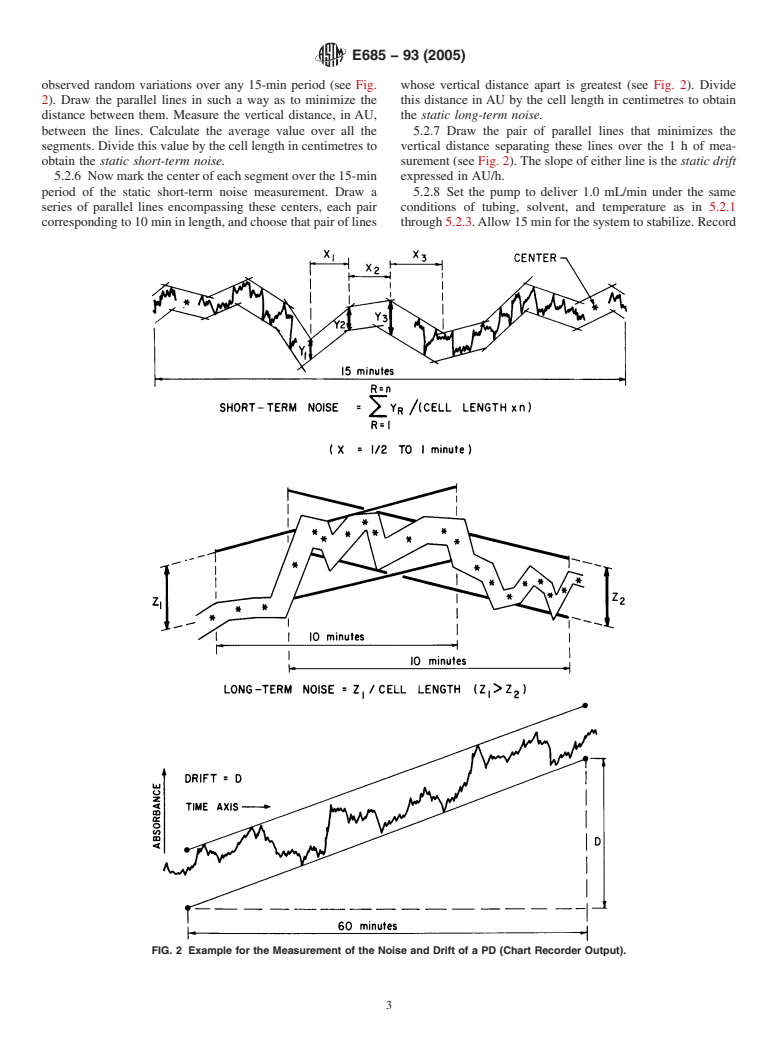
observed random variations over any 15-min period (see Fig. whose vertical distance apart is greatest (see Fig. 2). Divide
2). Draw the parallel lines in such a way as to minimize the this distance inAU by the cell length in centimetres to obtain
distance between them. Measure the vertical distance, in AU, the static long-term noise.
between the lines. Calculate the average value over all the 5.2.7 Draw the pair of parallel lines that minimizes the
segments.Dividethisvaluebythecelllengthincentimetresto vertical distance separating these lines over the1hof mea-
obtain the static short-term noise. surement (see Fig. 2).The slope of either line is the static drift
5.2.6 Nowmarkthecenterofeachsegmentoverthe15-min expressed in AU/h.
period of the static short-term noise measurement. Draw a 5.2.8 Set the pump to deliver 1.0 mL/min under the same
series of parallel lines encompassing these centers, each pair conditions of tubing, solvent, and temperature as in 5.2.1
correspondingto10mininlength,andchoosethatpairoflines through5.2.3.Allow15minforthesystemtostabilize.Record
FIG. 2 Example for the Measurement of the Noise and Drift of a PD (Chart Recorder Output).
E685 − 93 (2005)
at least1hof signal under these flowing conditions, during 6.1.3 Determine the minimum detectability (minimum de-
which time the ambient temperature should not change by tectable concentration) of the test substance by calculating the
more than 2°C. concentration that would correspond to twice the static short-
5.2.9 Draw pairs of parallel lines, measure the vertical term noise. Specify the solute and solvent.
distances, and calculate the dynamic short-term noise follow-
6.1.4 Calculatetheratiooftheupperlimitoflinearitytothe
ing the procedure of 5.2.5.
lower limit of linearity to give the linear range expressed as a
5.2.10 Make the measurement for the dynamic long-term
number. As this procedure is a worst case situation, the linear
noise following the procedure outlined in 5.2.6.
range may be expected to be greater for compounds having a
5.2.11 Draw the pair of parallel lines as directed in 5.2.7.
broad spectral band in the region of the chosen wavelength.
The slope of these lines is the dynamic drift.
6.1.5 Plot or calculate the detector response (AU) versus
5.2.12 The actual noise of the system may be larger or
concentrations (µg/mL) for a test substance of known molar
smaller than the observed values, depending upon the method
absorptivity to find the best-fit line through the origin. Calcu-
of data collection, or signal monitoring of the detector, since
late the molar absorptivity, ϵ, of the test solution as follows:
observed noise is a function of the frequency, speed of
slope 3MW
response, and bandwidth of the readout device.
ϵ 5 (1)
b
6. Minimum Detectability, Linear Range, and
where:
Calibration
slope = the slope of the linear portion of the plot,AU·µl/µg,
MW = molecular weight, g/mole, and
6.1 Methods of Measurement—For the determination of the
b = nominal cell length, cm, as specified by the
linearrangeofaPD, (12)foraspecificsubstance,theresponse
manufacturer.
to that test substance must be determined. The following
procedure is designed to provide a worst-case procedure.
Compare the value of ϵ obtained with an experimentally
6.1.1 Dissolve in methanol a suitable compound with an
determined value or one from the literature (Note 3). Should
ultraviolet spectral absorbance that changes rapidly at the
the values differ by more than 5%, the PD may require
wavelength of interest. Choose a concentration that is ex-
adjustment. Consult the manufacturer’s directions.
pected to exceed the linear range, typically to give an absor-
NOTE 3—For example, the values of molar absorptivity for uracil in
bance above 2AU. Dilute the solution accurately in a series to
3 3
methanol are 7.7×10 at 254 nm and 1.42×10 at 280 nm; for potassium
coverthelinearrange,thatis,downtotheminimumdetectable
dichromate in 0.01 N sulfuric acid they are 4.22×10 at 254 nm and
concentration. Rinse the sample cell with methanol and zero
3.60×10 at 280 nm.
the detector with methanol in the cell. Rinse the cell with the
solution of lowest concentration until a stable reading is
7. Response Time
obtained; usually rinsing the cell with 1 mL is sufficient.
7.1 The response time of the detector may become signifi-
Record the detector output. After rinsing the syringe thor-
cant when a short micro-particle column and a high-speed
oughly with the next more concentrated solution, fill the cell
recorderareused.Also,itispossible,byusinganintentionally
withthesolutionfromeachdilutioninturn.Obtainaminimum
slow response time, to reduce the observed noise and hence
of five on-scale measurements. Measure under static condi-
increase the apparent linear range. Although this would have
tions.
little effect on broad peaks, the signal from narrow peaks
6.1.2 Calculate the ratio of detector response (AU) to
would be significantly degraded. Measure at the highest and
concentration (µg/mL) for each solution and plot these ratios
lowest values of the electronic filter if it is variable.
versus log concentration (see Fig. 1). The region of linearity
will define a horizontal line of constant response ratio. At 7.2 Method of Measurement:
higher concentrations, there will typically be a negative devia-
7.2.1 The composition of the mobile phase is changed in a
tion from linearity, while at lower concentrations there may be
stepwise manner and the output signal is recorded on the
deviation in
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.