ASTM E685-93(2021)
(Practice)Standard Practice for Testing Fixed-Wavelength Photometric Detectors Used in Liquid Chromatography
Standard Practice for Testing Fixed-Wavelength Photometric Detectors Used in Liquid Chromatography
SIGNIFICANCE AND USE
4.1 Although it is possible to observe and measure each of the several characteristics of a detector under different and unique conditions, it is the intent of this practice that a complete set of detector specifications should be obtained under the same operating conditions. It should also be noted that to completely specify a detector’s capability, its performance should be measured at several sets of conditions within the useful range of the detector. The terms and tests described in this practice are sufficiently general that they may be used regardless of the ultimate operating parameters.
4.2 Linearity and response time of the recorder or other readout device used should be such that they do not distort or otherwise interfere with the performance of the detector. This requires adjusting the gain, damping, and calibration in accordance with the manufacturer's directions. If additional electronic filters or amplifiers are used between the detector and the final readout device, their characteristics should also first be established.
SCOPE
1.1 This practice is intended to serve as a guide for the testing of the performance of a photometric detector (PD) used as the detection component of a liquid-chromatographic (LC) system operating at one or more fixed wavelengths in the range 210 nm to 800 nm. Measurements are made at 254 nm, if possible, and are optional at other wavelengths.
1.2 This practice is intended to describe the performance of the detector both independently of the chromatographic system (static conditions) and with flowing solvent (dynamic conditions).
1.3 For general liquid chromatographic procedures, consult Refs (1-9).2
1.4 For general information concerning the principles, construction, operation, and evaluation of liquid-chromatography detectors, see Refs (10 and 11) in addition to the sections devoted to detectors in Refs (1-7).
1.5 This standard does not purport to address all of the safety problems, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.6 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: E685 − 93 (Reapproved 2021)
Standard Practice for
Testing Fixed-Wavelength Photometric Detectors Used in
Liquid Chromatography
This standard is issued under the fixed designation E685; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
2.1 ASTM Standards:
1.1 This practice is intended to serve as a guide for the
E275PracticeforDescribingandMeasuringPerformanceof
testingoftheperformanceofaphotometricdetector(PD)used
Ultraviolet and Visible Spectrophotometers
as the detection component of a liquid-chromatographic (LC)
E682Practice for Liquid Chromatography Terms and Rela-
systemoperatingatoneormorefixedwavelengthsintherange
tionships
210nm to 800 nm. Measurements are made at 254 nm, if
possible, and are optional at other wavelengths.
3. Terminology
1.2 This practice is intended to describe the performance of
3.1 Definitions:
thedetectorbothindependentlyofthechromatographicsystem
3.1.1 absorbance calibration, n—the procedure that verifies
(static conditions) and with flowing solvent (dynamic condi-
that the absorbance scale is correct within 65%.
tions).
3.1.2 drift, n—the average slope of the noise envelope
1.3 For general liquid chromatographic procedures, consult
expressed in absorbance units per hour (AU/h) as measured
Refs (1-9).
over a period of 1 h.
3.1.3 dynamic, n—under conditions of a flow rate of 1.0
1.4 For general information concerning the principles,
construction, operation, and evaluation of liquid- mL/min.
chromatography detectors, see Refs (10 and 11) in addition to
3.1.4 linear range, n—of a PD, the range of concentrations
the sections devoted to detectors in Refs (1-7).
of a test substance in a mobile phase over which the response
ofthedetectorisconstanttowithin5%asdeterminedfromthe
1.5 This standard does not purport to address all of the
linearity plot specified below and illustrated in Fig. 1. The
safety problems, if any, associated with its use. It is the
linear range should be expressed as the ratio of the highest
responsibility of the user of this standard to establish appro-
concentration to the minimum detectable concentration or the
priate safety, health, and environmental practices and deter-
lowest linear concentration, whichever is greatest.
mine the applicability of regulatory limitations prior to use.
3.1.5 long-term noise, n—the maximum amplitude in AU
1.6 This international standard was developed in accor-
for all random variations of the detector signal of frequencies
dance with internationally recognized principles on standard-
between 6cycles per hour and 60 cycles per hour (0.1cycles
ization established in the Decision on Principles for the
per min and 1.0 cycles per min).
Development of International Standards, Guides and Recom-
3.1.5.1 Discussion—Itrepresentsnoisethatcanbemistaken
mendations issued by the World Trade Organization Technical
for a late-eluting peak. This noise corresponds to the observed
Barriers to Trade (TBT) Committee.
noise only and may not always be present.
3.1.6 minimum detectability, n—of a PD, that concentration
1 ofaspecificsoluteinaspecificsolventthatresultsinadetector
This practice is under the jurisdiction ofASTM Committee E13 on Molecular
Spectroscopy and Separation Science and is the direct responsibility of Subcom- response corresponding to twice the static short-term noise.
mittee E13.19 on Separation Science.
Current edition approved April 1, 2021. Published April 2021. Originally
approved in 1979. Last previous edition approved in 2013 as E685–93(2013). For referenced ASTM standards, visit the ASTM website, www.astm.org, or
DOI: 10.1520/E0685-93R21. contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Theboldfacenumbersinparenthesesrefertothelistofreferencesattheendof Standards volume information, refer to the standard’s Document Summary page on
this practice. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E685 − 93 (2021)
otherwise interfere with the performance of the detector. This
requires adjusting the gain, damping, and calibration in accor-
dance with the manufacturer’s directions. If additional elec-
tronicfiltersoramplifiersareusedbetweenthedetectorandthe
final readout device, their characteristics should also first be
established.
5. Noise and Drift
5.1 Test Conditions—Pure, degassed methanol of suitable
grade shall be used in the sample cell.Air or nitrogen shall be
used in the reference cell if there is one. Nitrogen is preferred
where the presence of high-voltage equipment makes it likely
that there is ozone in the air. Protect the entire system from
temperature fluctuations because these will lead to detectable
drift.
5.1.1 The detector should be located at the test site and
turned on at least 24 h before the start of testing. Insufficient
warm-upmayresultindriftinexcessoftheactualvalueforthe
detector.
5.2 Methods of Measurement:
FIG. 1 Example of a Linearity Plot for a Photometric Detector
5.2.1 Connect a suitable device (Note 1) between the pump
and the detector to provide at least 75 kPa (500 psi) back
pressure at 1.0 mL/min flow of methanol. Connect a short
3.1.7 response time (speed of output), n—the detector, the
length(about100mm)of0.25mm(0.01in.)internal-diameter
timerequiredforthedetectoroutputtochangefrom10to90%
stainless steel tubing to the outlet tube of the detector to retard
of the new equilibrium value when the composition of the
bubble formation. Connect the recorder to the proper detector
mobilephaseischangedinastepwisemanner,withinthelinear
output channels.
range of the detector.
3.1.7.1 Discussion—Because the detector volume is very
NOTE 1—Suggested devices include (a)2mto4mof0.1mm
(0.004in.) internal-diameter stainless steel tubing, (b) about 250 mm of
small and the transport rate is not diffusion dependent, the
0.25mm to 0.5mm (0.01in. to 0.02in.) internal-diameter stainless steel
response time is generally fast enough to be unimportant. It is
tubingcrimpedwithpliersorcutters,or(c)aconstantback-pressurevalve
generally comparable to the response time of the recorder and
located between the pump and the injector.
dependent on the response time of the detector electrometer
5.2.2 Repeatedly rinse the reservoir and chromatographic
and on the recorder amplifier. Factors that affect the observed
system, including the detector, with degassed methanol to
response time include the true detector response time, elec-
remove from the system all other solvents, any soluble
tronic filtering, and system band-broadening.
material, and any entrained gasses. Fill the reservoir with
3.1.8 short-term noise, n—the maximum amplitude, peak to
methanolandpumpthissolventthroughthesystemforatleast
peak, inAU for all random variations of the detector signal of
30 min to complete the system cleanup.
a frequency greater than one cycle per minute.
5.2.3 Air or nitrogen is used in the reference cell, if any.
3.1.8.1 Discussion—Itdeterminesthesmallestsignaldetect-
Ensure that the cell is clean, free of dust, and completely dry.
able by a PD, limits the precision attainable in quantitation of
5.2.4 To perform the static test, cease pumping and allow
trace-level samples, and sets the lower limit on linearity. This
thechromatographicsystemtostabilizeforatleast1hatroom
noise corresponds to the observed noise only.
temperature without flow. Set the attenuator at maximum
3.1.9 static, n—under conditions of no flow.
sensitivity (lowest attenuation), that is, the setting for the
smallest value of absorbance units full-scale (AUFS). Adjust
4. Significance and Use
the response time as close as possible to 2 s for a PD that has
4.1 Although it is possible to observe and measure each of a variable response time (Note 2). Record the response time
the several characteristics of a detector under different and used.Adjustthedetectoroutputtonearmidscaleonthereadout
unique conditions, it is the intent of this practice that a device. Record at least1hof detector signal under these
complete set of detector specifications should be obtained conditions, during which time the ambient temperature should
under the same operating conditions. It should also be noted not change by more than 2°C.
that to completely specify a detector’s capability, its perfor-
NOTE2—Timeconstantisconvertedtoresponsetimebymultiplyingby
mance should be measured at several sets of conditions within
the factor 2.2. The effect of electronic filtering on observed noise may be
the useful range of the detector. The terms and tests described
studied by repeating the noise measurements for a series of response-time
settings.
in this practice are sufficiently general that they may be used
regardless of the ultimate operating parameters.
4.2 Linearity and response time of the recorder or other
Distilled-in-glass or liquid-chromatography grade. Complete freedom from
readout device used should be such that they do not distort or particles may require filtration, for example, through a 0.45µm membrane filter.
E685 − 93 (2021)
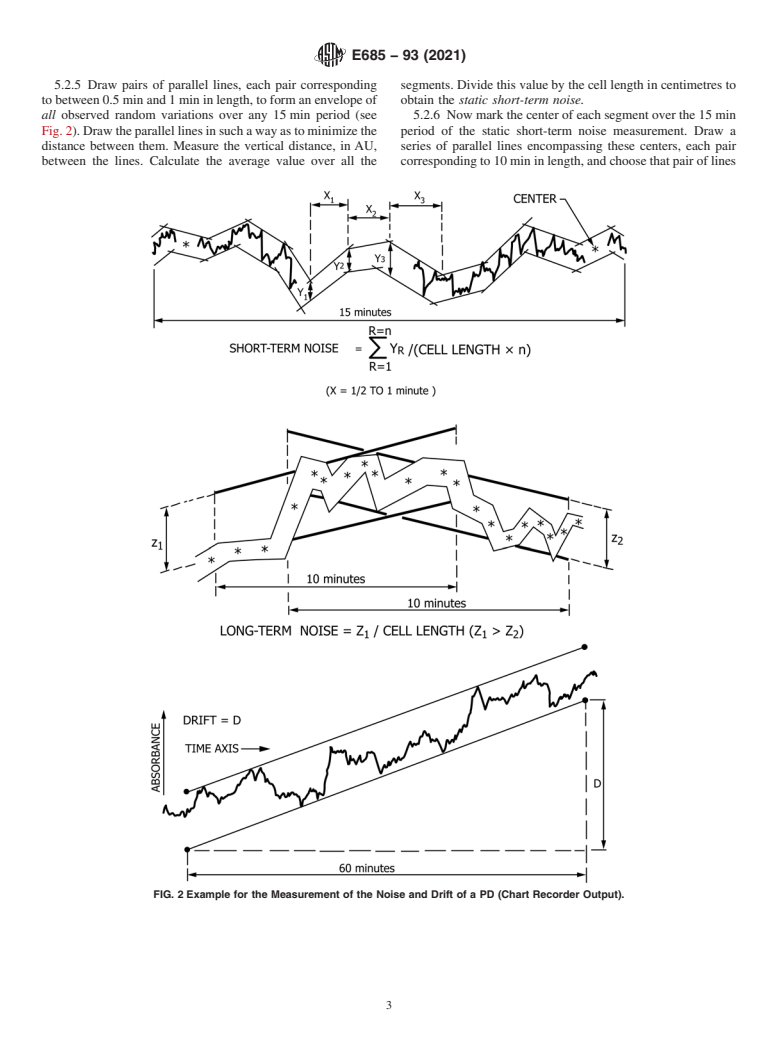
5.2.5 Draw pairs of parallel lines, each pair corresponding segments.Dividethisvaluebythecelllengthincentimetresto
tobetween0.5minand1mininlength,toformanenvelopeof obtain the static short-term noise.
all observed random variations over any 15min period (see 5.2.6 Nowmarkthecenterofeachsegmentoverthe15min
Fig.2).Drawtheparallellinesinsuchawayastominimizethe period of the static short-term noise measurement. Draw a
distance between them. Measure the vertical distance, in AU, series of parallel lines encompassing these centers, each pair
between the lines. Calculate the average value over all the correspondingto10mininlength,andchoosethatpairoflines
FIG. 2 Example for the Measurement of the Noise and Drift of a PD (Chart Recorder Output).
E685 − 93 (2021)
whose vertical distance apart is greatest (see Fig. 2). Divide deviation in either direction. Draw horizontal lines 5% above
this distance in AU by the cell length in centimetres to obtain and below the line of constant response ratio. The upper limit
the static long-term noise. of linearity is the concentration at which the line of measured
5.2.7 Draw the pair of parallel lines that minimizes the response ratio intersects one of the 5% bracketing lines at the
vertical distance separating these lines over the 1 h of mea- highconcentrationend.Thelowerlimitoflinearityiseitherthe
surement (see Fig. 2).The slope of either line is the static drift minimum detectable concentration (see 6.1.3) or the concen-
expressed in AU/h. tration at which the line of measured response ratio intersects
5.2.8 Set the pump to deliver 1.0 mL/min under the same one of the bracketing lines at the low concentration end,
conditions of tubing, solvent, and temperature as in 5.2.1 whichever is greater.
through5.2.3.Allow15minforthesystemtostabilize.Record
6.1.3 Determine the minimum detectability (minimum de-
at least1hof signal under these flowing conditions, during
tectable concentration) of the test substance by calculating the
which time the ambient temperature should not change by
concentration that would correspond to twice the static short-
more than 2°C.
term noise. Specify the solute and solvent.
5.2.9 Draw pairs of parallel lines, measure the vertical
6.1.4 Calculatetheratiooftheupperlimitoflinearitytothe
distances, and calculate the dynamic short-term noise follow-
lower limit of linearity to give the linear range expressed as a
ing the procedure of 5.2.5.
number. As this procedure is a worst case situation, the linear
5.2.10 Make the measurement for the dynamic long-term
range may be expected to be greater for compounds having a
noise following the procedure outlined in 5.2.6.
broad spectral band in the region of the chosen wavelength.
5.2.11 Draw the pair of parallel lines as directed in 5.2.7.
6.1.5 Plot or calculate the detector response (AU) versus
The slope of these lines is the dynamic drift.
concentrations (µg/mL) for a test substance of known molar
5.2.12 The actual noise of the system may be larger or
absorptivity to find the best-fit line through the origin. Calcu-
smaller than the observed values, depending upon the method
late the molar absorptivity, ε, of the test solution as follows:
of data collection, or signal monitoring of the detector, since
slope 3MW
observed noise is a function of the frequency, speed of
ε 5 (1)
b
response, and bandwidth of the readout device.
where:
6. Minimum Detectability, Linear Range, and
slope = the slope of the linear portion of the plot,AU·µl/µg,
Calibration
MW = molecular weight, g/mole, and
6.1 Methods of Measurement—For the determination of the
b = nominal cell length, cm, as specified by the
linearrangeofaPD, (12)foraspecificsubstance,theresponse
manufacturer.
to that test substance must be determined. The following
Compare the value of ε obtained with an experimentally
procedure is designed to provide a worst-case procedure.
determined value or one from the literature (Note 3). Should
6.1.1 Dissolve in methanol a suitable compound with an
the values differ by more than 5%, the PD may require
ultraviolet spectral absorbance that changes rapidly at the
adjustment. Consult the manufacturer’s directions.
wavelength of interest. Choose a concentration that is ex-
pected to exceed the linear range, typically to give an absor-
NOTE 3—For example, the values of molar absorptivity for uracil in
3 3
methanolare7.7×10 at254nmand1.42×10 at280nm;forpotassium
bance above 2AU. Dilute the solution accurately in a series to
dichromate in 0.01 N sulfuric acid they are 4.22×10 at 254 nm and
coverthelinearrange,thatis,downtotheminimumdetectable
3.60×10 at 280 nm.
concentration. Rinse the sample cell with methanol and zero
the detector with methanol in the cell. Rinse the cell with the
7. Response Time
solution of lowest concentration until a stable reading is
obtained; usually rinsing the cell with 1 mL is sufficient. 7.1 The response time of the detector may become signifi-
cant when a short micro-particle column and a high-speed
Record the detector output. After rinsing the syringe thor-
oughly with the next more concentrated solution, fill the cell recorderareused.Also,itispossible,byusinganintentionally
slow response time, to reduce
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.