ASTM E2304-03(2011)
(Practice)Standard Practice for Use of a LiF Photo-Fluorescent Film Dosimetry System (Withdrawn 2020)
Standard Practice for Use of a LiF Photo-Fluorescent Film Dosimetry System (Withdrawn 2020)
SIGNIFICANCE AND USE
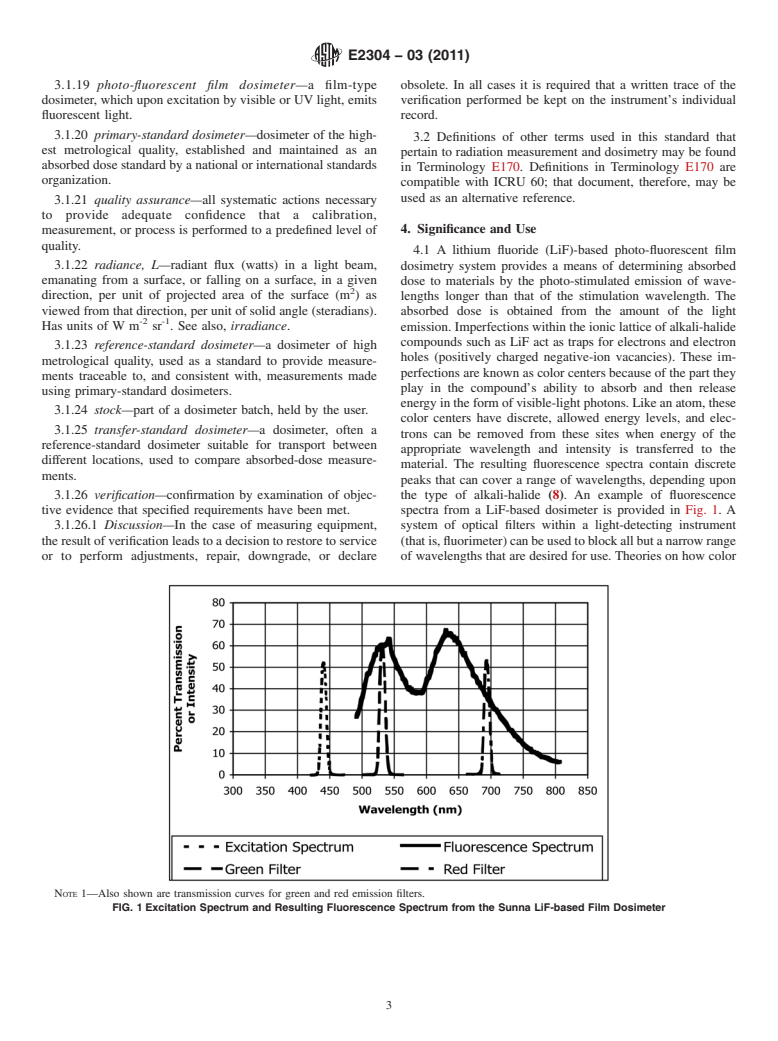
A lithium fluoride (LiF)-based photo-fluorescent film dosimetry system provides a means of determining absorbed dose to materials by the photo-stimulated emission of wavelengths longer than that of the stimulation wavelength. The absorbed dose is obtained from the amount of the light emission. Imperfections within the ionic lattice of alkali-halide compounds such as LiF act as traps for electrons and electron holes (positively charged negative-ion vacancies). These imperfections are known as color centers because of the part they play in the compound's ability to absorb and then release energy in the form of visible-light photons. Like an atom, these color centers have discrete, allowed energy levels, and electrons can be removed from these sites when energy of the appropriate wavelength and intensity is transferred to the material. The resulting fluorescence spectra contain discrete peaks that can cover a range of wavelengths, depending upon the type of alkali-halide (8). An example of fluorescence spectra from a LiF-based dosimeter is provided in Fig. 1. A system of optical filters within a light-detecting instrument (that is, fluorimeter) can be used to block all but a narrow range of wavelengths that are desired for use. Theories on how color centers are formed, how luminescence mechanisms work, and their application in dosimetry are found in Refs (8-13). For characterization studies on specific photo-fluorescent dosimeters see Refs (1-7) and (14-19).
In the application of a specific dosimetry system, absorbed dose is determined by use of an experimentally-derived calibration curve. The calibration curve for the photo-fluorescent dosimeter is the functional relationship between ΔEf and D, and is determined by measuring the net fluorescence of sets of dosimeters irradiated to known absorbed doses. These absorbed doses span the range of utilization of the system.
Photo-fluorescent dosimetry systems require calibration traceable to national standards. See ISO/A...
SCOPE
1.1 This practice covers the handling, testing, and procedure for using a lithium fluoride (LiF)-based photo-fluorescent film dosimetry system to measure absorbed dose (relative to water) in materials irradiated by photons or electrons. Other alkali halides that may also exhibit photofluorescence (for example, NaCl, NaF, and KCl) are not covered in this practice. Although various alkali halides have been used for dosimetry for years utilizing thermoluminescence, the use of photoluminescence is relatively new.
1.2 This practice applies to photo-fluorescent film dosimeters (referred hereafter as photo-fluorescent dosimeters) that can be used within part or all of the following ranges:
1.2.1 Absorbed dose range of 5 × 10-2 to 3 × 102 kGy (1-3).
1.2.2 Absorbed dose rate range of 0.3 to 2 × 104 Gy/s (2-5)).
1.2.3 Radiation energy range for photons of 0.05 to 10 MeV (2).
1.2.4 Radiation energy range for electrons of 0.1 to 10 MeV (2).
1.2.5 Radiation temperature range of -20 to +60°C (6,7).
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
WITHDRAWN RATIONALE
This practice covers the handling, testing, and procedure for using a lithium fluoride (LiF)-based photo-fluorescent film dosimetry system to measure absorbed dose (relative to water) in materials irradiated by photons or electrons.
Formerly under the jurisdiction of Committee E61 on Radiation Processing, this practice was withdrawn in January 2020 in accordance with section 10.6.3 of the Regulations Governing ASTM Technical Committees, which requires that standards shall be updated by the end...
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E2304 − 03 (Reapproved 2011)
Standard Practice for
Use of a LiF Photo-Fluorescent Film Dosimetry System
This standard is issued under the fixed designation E2304; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope E170Terminology Relating to Radiation Measurements and
Dosimetry
1.1 Thispracticecoversthehandling,testing,andprocedure
E275PracticeforDescribingandMeasuringPerformanceof
for using a lithium fluoride (LiF)-based photo-fluorescent film
Ultraviolet and Visible Spectrophotometers
dosimetry system to measure absorbed dose (relative to water)
E925Practice for Monitoring the Calibration of Ultraviolet-
in materials irradiated by photons or electrons. Other alkali
Visible Spectrophotometers whose Spectral Bandwidth
halides that may also exhibit photofluorescence (for example,
does not Exceed 2 nm
NaCl,NaF,andKCl)arenotcoveredinthispractice.Although
2.2 ISO/ASTM Standards:
various alkali halides have been used for dosimetry for years
51204Practice for Dosimetry in Gamma Irradiation Facili-
utilizing thermoluminescence, the use of photoluminescence is
ties for Food Processing
relatively new.
51261Guide for Selection and Calibration of Dosimetry
1.2 This practice applies to photo-fluorescent film dosim-
Systems for Radiation Processing
eters (referred hereafter as photo-fluorescent dosimeters) that
51431Practice for Dosimetry in Electron and Bremsstrahl-
can be used within part or all of the following ranges:
ung Irradiation Facilities for Food Processing
-2 2 2
1.2.1 Absorbeddoserangeof5×10 to3×10 kGy (1-3).
51608Practice for Dosimetry in an X-ray (Bremsstrahlung)
1.2.2 Absorbeddoseraterangeof0.3to2×10 Gy/s (2-5)).
Facility for Radiation Processing
1.2.3 Radiationenergyrangeforphotonsof0.05to10MeV
51649Practice for Dosimetry in an Electron Beam Facility
(2).
forRadiationProcessingatEnergiesbetween300keVand
1.2.4 Radiationenergyrangeforelectronsof0.1to10MeV
25 MeV
(2).
51702Practice for Dosimetry in a Gamma Irradiation Facil-
1.2.5 Radiation temperature range of -20 to +60°C (6,7).
ity for Radiation Processing
1.3 The values stated in SI units are to be regarded as
51707Guide for Estimating Uncertainties in Dosimetry for
standard. No other units of measurement are included in this
Radiation Processing
standard.
51818Practice for Dosimetry in an Electron Beam Facility
for Radiation Processing at Energies between 80 keV and
1.4 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the 300 keV
51956Practice for Thermoluminescence-Dosimetry (TLD)
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica- Systems for Radiation Processing
bility of regulatory limitations prior to use.
2.3 International Commission on Radiation Units and Mea-
surements (ICRU) Reports:
2. Referenced Documents
ICRU Report 14Radiation Dosimetry: X-rays and Gamma
rays with Maximum Photon Energies Between 0.6 and 50
2.1 ASTM Standards:
MeV
ICRU Report 17Radiation Dosimetry: X-rays Generated at
1 Potentials of 5 to 150 kV
This practice is under the jurisdiction of ASTM Committee E61 on Radiation
Processingand is the direct responsibility of Subcommittee E61.02 on Dosimetry ICRU Report 34The Dosimetry of Pulsed Radiation
Systems.
ICRU Report 35Radiation Dosimetry: Electron Beams with
Current edition approved Nov. 1, 2011. Published November 2011. Originally
Energies Between 1 and 50 MeV
approved in 2003. Last previous edition approved in 2003 as E2304-03. DOI:
ICRU Report 60Fundamental Quantities and Units for
10.1520/E2304-03R11.
Theboldfacenumbersinparenthesesrefertothelistofreferencesattheendof
Ionizing Radiation
this standard.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on Available from International Commission on Radiation Units and
the ASTM website. Measurements, 7910 Woodmont Ave., Suite 800, Bethesda, MD 20814, USA.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E2304 − 03 (2011)
3. Terminology in a single production run under controlled, consistent
conditions, and having a unique identification code.
3.1 Definitions:
3.1.8 dosimetry system—system used for determining ab-
3.1.1 absorbed dose, D—quantity of ionizing radiation en-
sorbed dose, consisting of dosimeters, measurement instru-
ergyimpartedperunitmassofaspecifiedmaterial.TheSIunit
ments and their associated reference standards, and procedures
of absorbed dose is the gray (Gy), where 1 gray is equivalent
for the system’s use.
to the absorption of 1 joule per kilogram of the specified
-1
material(1Gy=1Jkg ).Themathematicalrelationshipisthe
3.1.9 electron equilibrium—chargedparticleequilibriumfor
quotientof dεby dm,where dεisthemeanincrementalenergy
¯ ¯
electrons.
imparted by ionizing radiation to matter of incremental mass
3.1.10 fluorescence—one of the four main luminescence
dm (see ICRU 60).
mechanisms. In many materials, it involves the liberated
dε¯
electrons falling back to the valence band—directly or via a
D 5
dm
relaxationstate—tofillanelectronhole,resultingintherelease
3.1.1.1 Discussion—Absorbeddoseissometimesreferredto
ofaphoton.Inthecaseofalkali-halidestheliberatedelectrons
simply as dose. For a photon source under conditions of
donotfallbacktothevalanceband,butareexcitedtoahigher
charged particle-equilibrium, the absorbed dose, D, may be
state within the color center, and subsequently fall back to the
expressed as:
center’s ground state, resulting in the release of a photon.
µ
3.1.11 fluorescence signal, E—thephotometricreadingbya
en
f
D 5φE
ρ
spectrofluorimeter in terms of light intensity incident on the
photodetector. Typically, the value measured is some quantity
where:
proportional to the standardized quantity, irradiance, E (for
-2
i
φ = particle fluence (m ),
example, volts or amperes per unit area of detector surface, V
E = energy of the ionizing radiation (J), and
-2 -2
2 -1 cm orAcm ).
µ /ρ = mass energy absorption coefficient (m kg ).
en
3.1.12 fluorescence standard—asolidorliquidmaterialthat
Ifbremsstrahlungproductionwithinthespecifiedmaterialis
produces a fluorescence upon excitation, with an emitted
negligible, the mass energy absorption coefficient (µ /ρ)is
en
radiance that is calibrated and made traceable to a recognized
equal to the mass energy transfer coefficient (µ /ρ), and
tr
standard.
absorbeddoseisequaltokermaif,inaddition,charged-particle
3.1.13 fluorimeter—instrument used to measure the amount
equilibrium exists.
of fluorescence signal, E, emitted from a sample upon excita-
f
3.1.2 alkali halide—a binary compound consisting of a
tion by an energy source (usually in the form of light).
halogen (any of the five elements fluorine, chlorine, bromine,
3.1.14 irradiance, E—a radiometric term for the radiant
iodine, and astatine) and an alkali metal (for example, lithium,
i
-2
sodium, and potassium). fluxthatisincidentuponasurface,havingunitsofWm .Also
see radiance.
3.1.3 analysis wavelength—wavelength used in a spectro-
photometric instrument to help determine a desired dosimetric
NOTE 1—The standard symbol for irradiance is E; however, for this
quantity, for example, absorbed dose, by means of the mea- documentthesubscript, i,wasaddedtodistinguishirradiancefromenergy
of ionizing radiation (see 3.1.1) and fluorescence signal.
surement of optical absorbance, optical density, reflectance or
luminescence. 3.1.15 luminescence—photon emission from a solid or liq-
uid phosphor material during, or after, exposure to a form of
3.1.4 calibration facility—combinationofanionizingradia-
energy. The main luminescence mechanisms are fluorescence,
tion source and its associated instrumentation that provides a
phosphorescence, thermoluminescence, and photolumines-
uniformandreproducibleabsorbeddose,orabsorbed-doserate
cence.
traceable to national or international standards at a specified
locationandwithinaspecificmaterial,andthatmaybeusedto 3.1.16 measurement quality assurance plan—a documented
derive the dosimetry system’s response function or calibration program for the measurement process that ensures on a
curve. continuing basis that the overall uncertainty meets the require-
mentsofthespecificapplication.Thisplanrequirestraceability
3.1.5 charged-particle equilibrium—the condition that ex-
to, and consistency with, nationally or internationally recog-
ists in an incremental volume within a material under irradia-
nized standards.
tion if the kinetic energies and number of charged particles (of
each type) entering the volume are equal to those leaving the 3.1.17 measurement traceability—theabilitytodemonstrate
volume. by means of an unbroken chain of comparisons that a mea-
surement is in agreement within acceptable limits of uncer-
3.1.6 color center—imperfections (for example, negative-
taintywithcomparablenationallyorinternationallyrecognized
or positive-ion vacancies) within the ionic lattice of com-
standards.
pounds that have trapped electrons or electron holes. These
centers, upon excitation by energy in the form of light or heat, 3.1.18 net fluorescence,∆E—measuredfluorescencesignal,
f
can produce luminescence. E, from an irradiated sample, subtracted by the pre-irradiation
f
fluorescence, E , as follows:
o
3.1.7 dosimeter batch—quantity of dosimeters made from a
specific mass of material with uniform composition, fabricated ∆E 5 E 2 E
f f o
E2304 − 03 (2011)
3.1.19 photo-fluorescent film dosimeter—a film-type obsolete. In all cases it is required that a written trace of the
dosimeter, which upon excitation by visible or UVlight, emits verification performed be kept on the instrument’s individual
fluorescent light. record.
3.1.20 primary-standard dosimeter—dosimeter of the high-
3.2 Definitions of other terms used in this standard that
est metrological quality, established and maintained as an
pertain to radiation measurement and dosimetry may be found
absorbeddosestandardbyanationalorinternationalstandards
in Terminology E170. Definitions in Terminology E170 are
organization.
compatible with ICRU 60; that document, therefore, may be
used as an alternative reference.
3.1.21 quality assurance—all systematic actions necessary
to provide adequate confidence that a calibration,
4. Significance and Use
measurement, or process is performed to a predefined level of
quality.
4.1 A lithium fluoride (LiF)-based photo-fluorescent film
3.1.22 radiance, L—radiant flux (watts) in a light beam, dosimetry system provides a means of determining absorbed
emanating from a surface, or falling on a surface, in a given dose to materials by the photo-stimulated emission of wave-
direction, per unit of projected area of the surface (m)as
lengths longer than that of the stimulation wavelength. The
viewed from that direction, per unit of solid angle (steradians). absorbed dose is obtained from the amount of the light
-2 -1
Has units of W m sr . See also, irradiance.
emission.Imperfectionswithintheioniclatticeofalkali-halide
compounds such as LiF act as traps for electrons and electron
3.1.23 reference-standard dosimeter—a dosimeter of high
holes (positively charged negative-ion vacancies). These im-
metrological quality, used as a standard to provide measure-
perfectionsareknownascolorcentersbecauseofthepartthey
ments traceable to, and consistent with, measurements made
play in the compound’s ability to absorb and then release
using primary-standard dosimeters.
energyintheformofvisible-lightphotons.Likeanatom,these
3.1.24 stock—part of a dosimeter batch, held by the user.
color centers have discrete, allowed energy levels, and elec-
3.1.25 transfer-standard dosimeter—a dosimeter, often a
trons can be removed from these sites when energy of the
reference-standard dosimeter suitable for transport between
appropriate wavelength and intensity is transferred to the
different locations, used to compare absorbed-dose measure-
material. The resulting fluorescence spectra contain discrete
ments.
peaks that can cover a range of wavelengths, depending upon
3.1.26 verification—confirmation by examination of objec- the type of alkali-halide (8). An example of fluorescence
tive evidence that specified requirements have been met. spectra from a LiF-based dosimeter is provided in Fig. 1.A
3.1.26.1 Discussion—In the case of measuring equipment, system of optical filters within a light-detecting instrument
theresultofverificationleadstoadecisiontorestoretoservice (thatis,fluorimeter)canbeusedtoblockallbutanarrowrange
or to perform adjustments, repair, downgrade, or declare of wavelengths that are desired for use.Theories on how color
NOTE 1—Also shown are transmission curves for green and red emission filters.
FIG. 1 Excitation Spectrum and Resulting Fluorescence Spectrum from the Sunna LiF-based Film Dosimeter
E2304 − 03 (2011)
centers are formed, how luminescence mechanisms work, and proper excitation and emission filters, and light detector of
their application in dosimetry are found in Refs (8-13). For proper wavelength sensitivity, are utilized.
characterization studies on specific photo-fluorescent dosim-
5.1.3 Holder, to position the dosimeter reproducibly in the
eters see Refs (1-7) and (14-19).
path between the excitation source and detector.
4.2 In the application of a specific dosimetry system, 5.1.4 Fluorescence Standard, of the appropriate
absorbed dose is determined by use of an experimentally- wavelength, if available (see 7.9.6), to be used during system
derived calibration curve. The calibration curve for the photo- calibration and for periodic checks on the stability of the
fluorescent dosimeter is the functional relationship between fluorimeter response (that is, stability of excitation light and
∆E and D, and is determined by measuring the net fluores- light detector).
f
cenceofsetsofdosimetersirradiatedtoknownabsorbeddoses.
NOTE2—Publishedliteratureshouldprovidetheperiodofusefulnessof
These absorbed doses span the range of utilization of the
the fluorescence standard under typical conditions of use, and any
system.
certificate of calibration should include an expiration date.
4.3 Photo-fluorescent dosimetry systems require calibration
5.1.5 Calibrated Laboratory Oven, as appropriate, with a
traceable to national standards. See ISO/ASTM Guide 51261.
temperatur
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.