ASTM D1826-94(2010)
(Test Method)Standard Test Method for Calorific (Heating) Value of Gases in Natural Gas Range by Continuous Recording Calorimeter
Standard Test Method for Calorific (Heating) Value of Gases in Natural Gas Range by Continuous Recording Calorimeter
SIGNIFICANCE AND USE
This test method provides an accurate and reliable method to measure the total calorific value of a fuel gas, on a continuous basis, which is used for regulatory compliance, custody transfer, and process control.
SCOPE
1.1 This test method covers the determination with the continuous recording calorimeter (Note 1) of the total calorific (heating) value of fuel gas produced or sold in the natural gas range from 900 to 1200 Btu/standard ft3.
Note 1—An extensive investigation of the accuracy of the Cutler-Hammer recording gas calorimeter, when used with gases of high heating value, was made by the National Bureau of Standards in 1957 under a research project sponsored by the American Gas Association.
1.2 The subjects covered in this test method appear in the following sections:
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D1826 −94 (Reapproved 2010)
Standard Test Method for
Calorific (Heating) Value of Gases in Natural Gas Range by
Continuous Recording Calorimeter
This standard is issued under the fixed designation D1826; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2.1.2 British Thermal Unit, or Btu—is the defined Interna-
tional Tables British thermal unit (symbol Btu).
1.1 This test method covers the determination with the
continuous recording calorimeter (Note 1) of the total calorific
NOTE 2—The defining relationships are:
−1 −1
(heating) value of fuel gas produced or sold in the natural gas (a) 1 Btu·lb =2.326 J·g (exact)
(b) 1 lb=453.59237 g (exact).
range from 900 to 1200 Btu/standard ft .
By these relationships, 1 Btu = 1 055.05585262 J (exact). For most
NOTE 1—An extensive investigation of the accuracy of the Cutler- purposes, the value rounded to 1 Btu = 1 055.056 J is adequate.
Hammer recording gas calorimeter, when used with gases of high heating
2.1.3 combustion air—airusedforcombustion,atotalofthe
value, was made by the National Bureau of Standards in 1957 under a
portion mixed with the gas as primary air and the air supplied
research project sponsored by the American Gas Association.
around the burner tube as secondary air (theoretical air plus
1.2 The subjects covered in this test method appear in the
excess air).
following sections:
2.1.4 flue gases—the products, of combustion remaining in
Sections
the gaseous state, together with any excess air.
Air-Gas Ratio Test 11
Apparatus 5
2.1.5 heat-absorbing air—the heat exchange medium used
Basis of Measurement 14
to absorb the heat of combustion derived from the burning of
Cold Balance Test 10
Compensation of Complicating Factors 13
gaseous fuel.
Condition of Gas Sample 7
2.1.6 saturated basis—the expressed total calorific value of
Definitions 2
Installation of Apparatus 6
a gas when it is saturated with water vapor at standard
Maintenance Appendix X1 3
temperature and pressure; 1 ft of this gas is equivalent in dry
Operating Precautions Appendix X2
gascontentto0.9826ft ofdrygasatthestandardtemperature
Operation and Checking of Apparatus 9
Precision 15
of 60°F and standard pressure of 14.73 psia.
Scope 1
Significance and Use 4
NOTE 3—The definitions given in 2.1.6 and 2.1.10 are for total calorific
Standardization of Calorimeter 12
(heating) values per standard cubic foot of gas. The definitions corre-
Standardization, Preliminary, of Calorimeter by Hydrogen 8
sponding to any other unit quantity of gas are obtained by substituting the
Summary of Test Method 3
name of the desired unit in place of the term “standard cubic foot” in the
1.3 This standard does not purport to address all of the
definitions. Methods of calculating calorific (heating) values per cubic
foot of gas under any desired conditions of pressure, temperature, and
safety concerns, if any, associated with its use. It is the
water vapor content are specified in Section 14.
responsibility of the user of this standard to establish appro-
2.1.7 standard cubic foot of gas—the quantity of any gas
priate safety and health practices and determine the applica-
that at standard temperature and under standard pressure will
bility of regulatory limitations prior to use.
fill a space of 1 ft when in equilibrium with liquid water.
2. Terminology
2.1.8 standard pressure—is 14.73 psia.
2.1 Definitions of Terms Specific to This Standard:
NOTE 4—This is the pressure base adopted by the American National
2.1.1 The most important terms used in connection with the
Standards Institute in 1969 (Z132.1). According to Dalton’s law, this is
determination of the calorific value of gaseous fuels in record-
equivalent to stating that the partial pressure of the gas is:
ing calorimetry are as follows:
14.73−0.25636=14.47364 psia
where 0.25636 is the vapor pressure of water in psia at 60°F.
2.1.9 standard temperature—60°F, based on the interna-
ThistestmethodisunderthejurisdictionofASTMCommitteeD03onGaseous
tional practical temperature scale of 1968.
Fuels and is the direct responsibility of Subcommittee D03.03 on Determination of
Heating Value and Relative Density of Gaseous Fuels.
2.1.10 total calorific value (gross heating value, higher
CurrenteditionapprovedMay1,2010.PublishedJuly2010.Originallyapproved
heating value)—of a gas is the number of British thermal units
in 1961. Last previous edition approved in 2003 as D1826–94(2003). DOI:
10.1520/D1826-94R10. evolved by the complete combustion at constant pressure of
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D1826−94 (2010)
one standard cubic foot of gas with air, the temperature of the measured; and the recording unit which translates the heat
gas, air, and products of combustion being 60°F, and all the measurements into an indication of calorific (heating) value
water formed by the combustion reaction being condensed to and records it graphically on a strip chart recorder or digitally
the liquid state. if the new SMART-CAL is used (Note 6).
NOTE 5—The previous specified pressure base was the absolute
3. Summary of Test Method
pressure of a column of pure mercury 30 in. in height at 32°F and under
standard gravity (32.174 ft/s ). This is equivalent to 14.7346 psia.
3.1 The heating value is determined by imparting all of the
NOTE 6—Refer to specific manufacturer’s manual for pictures of the
heat obtained from the combustion of the test gas to a stream
recorder or the SMART-CAL, a digital indicating or printing device,
of air and measuring the rise in temperature of the air. The
currently used on new or retrofitted calorimeters.
streams of test gas and heat absorbing air are maintained in
fixed volumetric proportion to each other by metering devices
6. Installation of Apparatus
similar to the ordinary wet test meters geared together and
6.1 To secure the precise results that are possible with the
driven from a common electric motor.The meters are mounted
recording calorimeter, it is important that the instrument be
in a tank of water, the level of which is maintained and the
installed so that the surrounding conditions will not introduce
temperature of which determines the temperature of the enter-
errors. In general, more precise results will be secured when a
ing gas and air.
narrow range is maintained on the various conditions of the
3.2 The flue gas resulting from combustion of the gas
calorimeter environment.
(combustion products plus excess combustion air) is kept
6.2 Calorimeter Room—A typical installation of a single
separate from the heat-absorbing air and is cooled to a few
recording calorimeter is shown in Fig. 4. The detailed require-
degrees above the initial temperature of gas and air. The water
ments for the calorimeter room are given in Table 1.
formed in the combustion is practically all condensed to the
liquidstate.Consequently,thetemperatureriseproducedinthe
NOTE 7—Adetailed discussion of these requirements is included in the
heat-absorbing air is directly proportional to the heating value
latest edition of the manufacturer’s instruction book covering the record-
ing calorimeter. The information can be applied to all models of the
of the gas. Since all the heat from the combustion of the test
instrument.
gas sample, including the latent heat of vaporization of the
NOTE 8—The dimensions shown in Fig. 4 are for the latest model
water vapor formed in the combustion, is imparted to the
calorimeter.
heat-absorbing air, the calorimeter makes a direct determina-
6.3 Gas Connection—Locate the sample line that brings the
tionoftotalheatingvalue.Thetemperatureriseismeasuredby
gas to be tested to the calorimeter tank unit so that the heating
nickel resistance thermometers and is translated into Btu per
valueisactuallyrepresentativeoftheconditionsexistinginthe
standard cubic foot.
main gas line. Keep the sample line time lag as small as
possible by (1) locating the calorimeter tank unit close to the
4. Significance and Use
sample point, (2) running the sample line of small size pipe
4.1 This test method provides an accurate and reliable
(Note 9), and (3) operating the sample line at low pressure.
method to measure the total calorific value of a fuel gas, on a
Provideanadditionalpurgeburnerorableedtoalowpressure
continuous basis, which is used for regulatory compliance,
point.
custody transfer, and process control.
NOTE 9—Time lag may be calculated on the basis that the calorimeter
5. Apparatus uses about 1.2 ft /h.
5.1 The recording calorimeter (Note 5) consists of two 6.4 Electrical Wiring—The four leads for the resistance
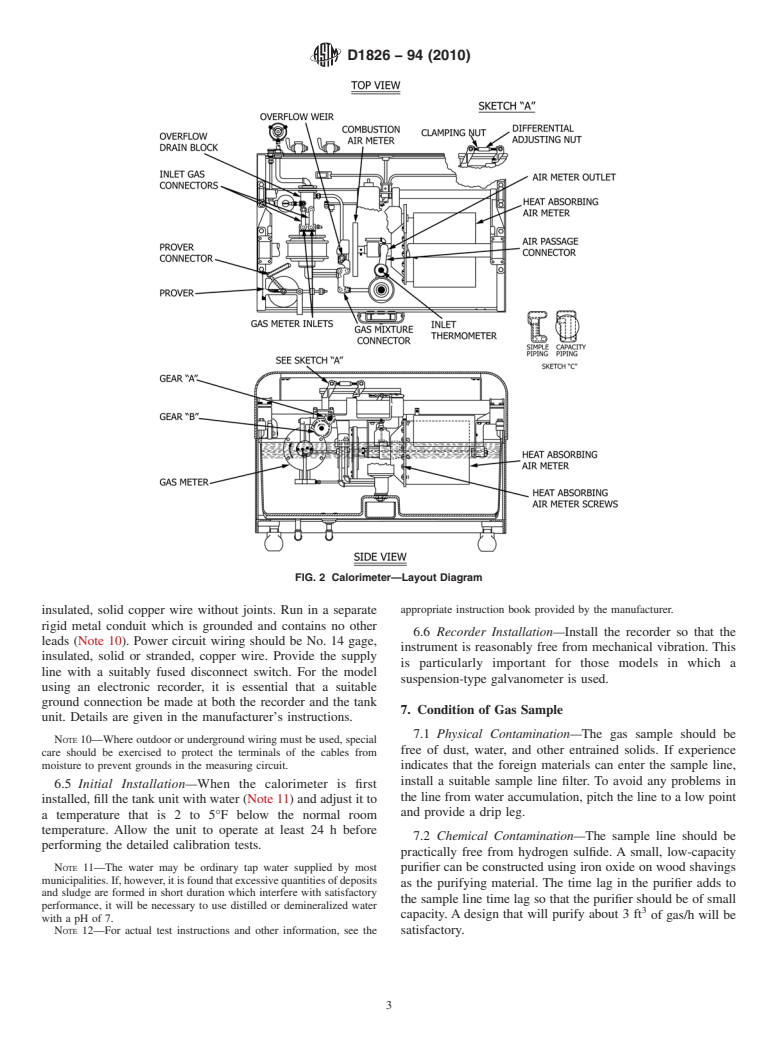
major units; the tank unit or calorimeter proper, Fig. 1, Fig. 2, thermometers between either the recorder or the Smart-Cal
and Fig. 3, in which the heating value of the test gas sample is junction box and the tank unit shall be of No. 12 gage,
FIG. 1 Calorimeter—Schematic Flow Diagram
D1826−94 (2010)
FIG. 2 Calorimeter—Layout Diagram
appropriate instruction book provided by the manufacturer.
insulated, solid copper wire without joints. Run in a separate
rigid metal conduit which is grounded and contains no other
6.6 Recorder Installation—Install the recorder so that the
leads (Note 10). Power circuit wiring should be No. 14 gage,
instrument is reasonably free from mechanical vibration. This
insulated, solid or stranded, copper wire. Provide the supply
is particularly important for those models in which a
line with a suitably fused disconnect switch. For the model
suspension-type galvanometer is used.
using an electronic recorder, it is essential that a suitable
ground connection be made at both the recorder and the tank
7. Condition of Gas Sample
unit. Details are given in the manufacturer’s instructions.
7.1 Physical Contamination—The gas sample should be
NOTE 10—Where outdoor or underground wiring must be used, special
free of dust, water, and other entrained solids. If experience
care should be exercised to protect the terminals of the cables from
moisture to prevent grounds in the measuring circuit. indicates that the foreign materials can enter the sample line,
install a suitable sample line filter. To avoid any problems in
6.5 Initial Installation—When the calorimeter is first
the line from water accumulation, pitch the line to a low point
installed, fill the tank unit with water (Note 11) and adjust it to
and provide a drip leg.
a temperature that is 2 to 5°F below the normal room
temperature. Allow the unit to operate at least 24 h before
7.2 Chemical Contamination—The sample line should be
performing the detailed calibration tests.
practically free from hydrogen sulfide. A small, low-capacity
NOTE 11—The water may be ordinary tap water supplied by most
purifier can be constructed using iron oxide on wood shavings
municipalities.If,however,itisfoundthatexcessivequantitiesofdeposits
as the purifying material. The time lag in the purifier adds to
and sludge are formed in short duration which interfere with satisfactory
the sample line time lag so that the purifier should be of small
performance, it will be necessary to use distilled or demineralized water
capacity. A design that will purify about 3 ft of gas/h will be
withapHof7.
NOTE 12—For actual test instructions and other information, see the satisfactory.
D1826−94 (2010)
FIG. 3 Calorimeter Combustion Chamber
NOTE 1—For each additional calorimeter at least 50% additional space is required; for example, for two calorimeters the room should be 12 by 18
ft inside; for three calorimeters 15 by 18 ft.
FIG. 4Calorimeter Room
D1826−94 (2010)
TABLE 1 Calorimeter Room Requirements
Detail Requirements
Space 1000 ft, min.
Ceiling height 8 ft, min.
Side wall widths 10 and 13 ft, min.
Windows One, on side normally away from sun (in northern hemisphere, the northern side).
Doors One, wth 3-ft opening, not in window wall. A door check is desirable.
Ventilation Natural ventilation using ceiling vent and a vent at floor level. Both should be located away from the tank unit.
Tank location The tank unit should be in a draft-free location with respect to heating and cooling units and natural ventilation.
Heating and cooling Controlled in the range 60 to 75°F with a variation of not more than 2.5°F from the set point.
Foundation floor The calorimeter should remain level at all times. Design for 3000-lb static and dynamic load. The tank feet should be on load
bearing parts of the floor.
Lighting No direct sunlight permitted on calorimeter tank unit.
Condition of air Essentially free from dust and absolutely free from any combustible gas for both measurement accuracy and safety. Trace
hydrocarbons can be removed from combustion air using a Hoskins furnace and a combustion air meter hood.
Vibration No vibrations or shocks shall be transmitted to the tank unit.
Water Pure pH-7 clean water shall be available for filling the tank and replenishing the reserve tank.
Power supply 115 V, 1 phase, 60 Hz, 1000 W for small motors. Lighting, heating, and cooling in addition.
1 1
Gas supply Sample pipe shall be ⁄4-in. tubing. Pressure shall be cut at the pipeline to 1 ⁄2 to 2 psig for minimum time lag. Pressure at the
calorimeter shall be 6 to 30 in. w.c.
Water supply and drain Desirable but not essential.
Radiation Tank unit shall be shielded from any hot radiation surfaces.
Safety It should be remembered that the calorimeter has open flames. Natural ventilation is sufficient in nonhazardous locations and
where only the aforementioned ⁄4-in. tubing service for natural gas at 1 psig is used. Hydrocarbon vapor detectors and purging
means should be considered for installations where location can be hazardous, where higher pressure gas is present, or where
gases heavier than air are involved. In all installations, lighting installations should be suitable for Division I, and incoming power
from underground services should have sealoffs.
8. Preliminary Standardization of Calorimeter by mentofanyparts,andtheestablishmentofaregularinspection
Hydrogen will ensure that the high degree of precision attainable will be
maintained. The manufacturer’s appropriate instruction book
8.1 The use of preliminary standardization by hydrogen test
givesdetailsoftheprocedureforoperatingtheinstrument.The
gasbeforetheuseofstandardmethaneatthetimeoftheinitial
following points should be checked periodically:
installation or after any comp
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.