ASTM D6238-98(2017)
(Test Method)Standard Test Method for Total Oxygen Demand in Water
Standard Test Method for Total Oxygen Demand in Water
SIGNIFICANCE AND USE
5.1 The measurement of oxygen demand parameters is critical to the control of process wastewaters. Biochemical oxygen demand (BOD) and chemical oxygen demand (COD) analyzers have long time cycles and in the case of COD analyzers use corrosive reagents with the inherent problem of disposal. Total oxygen demand analysis is faster, approximately 3 min, and uses no liquid reagents in its analysis.
5.2 TOD can be correlated to both COD and BOD, providing effective on-line control.
5.3 TOD offers several features which make it a more attractive measurement than carbon monitoring using total carbon (TC) or total organic carbon (TOC) analyzers. TOD is unaffected by the presence of inorganic carbon. TOD analysis will also indicate noncarbonaceous materials that consume or contribute oxygen. For example, the oxygen demand of ammonia, sulfite and sulfides will be reflected in the TOD measurement. Also, since the actual measurement is oxygen consumption, TOD reflects the oxidation state of the chemical compound (that is, urea and formic acid have the same number of carbon atoms, yet urea has five times the oxygen demand of formic acid).
SCOPE
1.1 This test method covers the determination of total oxygen demand in the range from 100 to 100 000 mg/L, in water and wastewater including brackish waters and brines (see 6.5). Larger concentrations, or samples with high suspended solids, or both, may be determined by suitable dilution of the sample.
1.1.1 Since the analysis is based on the change in oxygen reading of the carrier gas compared to that when a sample is introduced (see 4.1), the measurement range is a function of the amount of oxygen in the carrier gas. The higher the desired concentration range, the more oxygen required in the carrier gas. Under recommended conditions, the carrier gas concentration should be between two to four times the maximum desired oxygen demand.
1.1.2 The lower measurement range is limited by the stability of the baseline oxygen detector output. This signal is a function of the permeation system temperature, carrier gas flow rate, oxygen detector temperature, and reference sensor voltage. Combined, these variables limit the minimum recommended range to 2 to 100 mg/L.
1.1.3 The upper measurement range is limited by the maximum oxygen concentration in the carrier gas (100 %). With the recommended conditions of carrier gas concentration being two to four times the maximum oxygen demand, this limits the maximum possible oxygen demand to between 250 000 to 500 000 mg/L. However, as a practical application to water analysis, this test method will consider a maximum range of 100 000 mg/L.
1.2 This test method is applicable to all oxygen-demanding substances under the conditions of the test contained in the sample that can be injected into the reaction zone. The injector opening limits the maximum size of particles that can be injected. If oxygen-demanding substances that are water-insoluble liquids or solids are present, a preliminary treatment may be desired. These pretreatment methods are described in Annex A2.
1.3 This test method is particularly useful for measuring oxygen demand in certain industrial effluents and process streams. Its application for monitoring secondary sewage effluents is not established. Its use for the monitoring of natural waters is greatly limited by the interferences defined in Section 6.
1.4 In addition to laboratory analysis, this test method is applicable to on-stream monitoring. Sample conditioning techniques for solids pretreatment applications are noted in Annex A2.
1.5 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices a...
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: D6238 − 98 (Reapproved 2017)
Standard Test Method for
Total Oxygen Demand in Water
This standard is issued under the fixed designation D6238; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 1.3 This test method is particularly useful for measuring
oxygen demand in certain industrial effluents and process
1.1 This test method covers the determination of total
streams. Its application for monitoring secondary sewage
oxygen demand in the range from 100 to 100 000 mg/L, in
effluents is not established. Its use for the monitoring of natural
waterandwastewaterincludingbrackishwatersandbrines(see
waters is greatly limited by the interferences defined in Section
6.5). Larger concentrations, or samples with high suspended
6.
solids, or both, may be determined by suitable dilution of the
sample. 1.4 In addition to laboratory analysis, this test method is
1.1.1 Since the analysis is based on the change in oxygen applicable to on-stream monitoring. Sample conditioning tech-
reading of the carrier gas compared to that when a sample is niques for solids pretreatment applications are noted in Annex
introduced(see4.1),themeasurementrangeisafunctionofthe A2.
amount of oxygen in the carrier gas. The higher the desired
1.5 The values stated in SI units are to be regarded as
concentration range, the more oxygen required in the carrier
standard. No other units of measurement are included in this
gas. Under recommended conditions, the carrier gas concen-
standard.
tration should be between two to four times the maximum
1.6 This standard does not purport to address all of the
desired oxygen demand.
safety concerns, if any, associated with its use. It is the
1.1.2 The lower measurement range is limited by the
responsibility of the user of this standard to establish appro-
stability of the baseline oxygen detector output. This signal is
priate safety, health, and environmental practices and deter-
a function of the permeation system temperature, carrier gas
mine the applicability of regulatory limitations prior to use.
flow rate, oxygen detector temperature, and reference sensor
1.7 This international standard was developed in accor-
voltage. Combined, these variables limit the minimum recom-
dance with internationally recognized principles on standard-
mended range to 2 to 100 mg/L.
ization established in the Decision on Principles for the
1.1.3 The upper measurement range is limited by the
Development of International Standards, Guides and Recom-
maximum oxygen concentration in the carrier gas (100 %).
mendations issued by the World Trade Organization Technical
With the recommended conditions of carrier gas concentration
Barriers to Trade (TBT) Committee.
being two to four times the maximum oxygen demand, this
limits the maximum possible oxygen demand to between
2. Referenced Documents
250 000 to 500 000 mg⁄L. However, as a practical application
2.1 ASTM Standards:
to water analysis, this test method will consider a maximum
D888 Test Methods for Dissolved Oxygen in Water
range of 100 000 mg/L.
D1129 Terminology Relating to Water
1.2 This test method is applicable to all oxygen-demanding
D1192 Guide for Equipment for Sampling Water and Steam
substances under the conditions of the test contained in the 3
in Closed Conduits (Withdrawn 2003)
sample that can be injected into the reaction zone. The injector
D1193 Specification for Reagent Water
opening limits the maximum size of particles that can be
D2777 Practice for Determination of Precision and Bias of
injected. If oxygen-demanding substances that are water-
Applicable Test Methods of Committee D19 on Water
insoluble liquids or solids are present, a preliminary treatment
D3370 Practices for Sampling Water from Closed Conduits
may be desired. These pretreatment methods are described in
D3856 Guide for Management Systems in Laboratories
Annex A2.
Engaged in Analysis of Water
1 2
This test method is under the jurisdiction of ASTM Committee D19 on Water For referenced ASTM standards, visit the ASTM website, www.astm.org, or
andisthedirectresponsibilityofSubcommitteeD19.06onMethodsforAnalysisfor contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Organic Substances in Water. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved Dec. 15, 2017. Published January 2018. Originally the ASTM website.
approved in 1998. Last previous edition approved in 2011 as D6238 – 98 (2011). The last approved version of this historical standard is referenced on
DOI: 10.1520/D6238-98R17. www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D6238 − 98 (2017)
D5789 Practice for Writing Quality Control Specifications 5.3 TOD offers several features which make it a more
for Standard Test Methods for Organic Constituents attractive measurement than carbon monitoring using total
(Withdrawn 2002)
carbon (TC) or total organic carbon (TOC) analyzers. TOD is
D5847 Practice for Writing Quality Control Specifications
unaffected by the presence of inorganic carbon. TOD analysis
for Standard Test Methods for Water Analysis
will also indicate noncarbonaceous materials that consume or
contribute oxygen. For example, the oxygen demand of
3. Terminology
ammonia, sulfite and sulfides will be reflected in the TOD
measurement. Also, since the actual measurement is oxygen
3.1 Definitions:
consumption, TOD reflects the oxidation state of the chemical
3.1.1 For definitions of terms used in this standard, refer to
compound (that is, urea and formic acid have the same number
Terminology D1129.
of carbon atoms, yet urea has five times the oxygen demand of
3.2 Definitions of Terms Specific to This Standard:
formic acid).
3.2.1 total oxygen demand (TOD), n—theamountofoxygen
required to convert the elements in compounds to their most
6. Interferences
stable oxidized forms.
6.1 The dissolved oxygen concentrations will contribute a
4. Summary of Test Method
maximum error of 8 ppm. This error is only significant on
ranges below 0 to 100 ppm when samples have no dissolved
4.1 The total oxygen demand (TOD) measurement is
oxygen (DO) content. When operating in this range and
achievedbycontinuousanalysisoftheconcentrationofoxygen
samples contain low DO concentrations then compensation
in a combustion process gas effluent. The decrease in oxygen
may be necessary. Measure the DO in both solutions in
resulting from introduction of the sample into the combustion
accordance with Test Methods D888.Adjust the TOD result as
zone is a measure of oxygen demand.
follows: If DO of the sample is less than in the standard,
4.2 The oxidizable components in a liquid sample intro-
subtract DO variation. If DO of the sample is greater than in
duced into a carrier gas stream containing a fixed amount of
the standard, add DO variation to the TOD result.
oxygen flowing through a 900°C combustion tube are con-
verted to their stable oxides. The momentary reduction in the
6.2 Sulfuric acid will normally decompose under sample
oxygen concentration in the carrier gas is detected by an
combustion conditions as follows:
oxygen sensor and indicated on a digital display or recorded.
900°C 1
H SO H O1SO 1 O (1)
2 4 2 2 2
4.3 The TOD for the sample is obtained by comparing the
Catalyst 2
peak height to a calibration curve of peak heights for TOD
The oxygen release will result in a reduction in the TOD
standard solutions. The TOD for the standard solution is based
reading. However, alkali metal sulfates (that is, sodium and
on experimentally observed reactions in which carbon is
potassium salts) do not decompose under the combustion
converted to carbon dioxide, hydrogen to water, combined
conditions. If sulfates are present in the samples, adjust to pH
nitrogen including ammonia to nitric oxide, and elemental or
11 with NaOH prior to analysis.
organic sulfur to sulfur dioxide. Sample injection is achieved
by means of an automatic valve, that provides unattended
6.3 Nitrate salts decompose under sample combustion con-
multiple sampling in the laboratory or on-stream monitoring.
ditions as follows:
4.4 For monitoring applications, pretreatment of the sample
900°C 1
may be required. However, no single instruction can be written 2 NaNO Na O12NO11 O (2)
3 2 2
Catalyst 2
since pretreatment steps will be a function of the specific
characteristics of the sample stream.
The resulting generation of oxygen reduces the oxygen
demand.
5. Significance and Use
6.4 Heavy metal ions have been reported to accumulate in
5.1 The measurement of oxygen demand parameters is
the system resulting in a significant loss of sensitivity. The
critical to the control of process wastewaters. Biochemical
history of the combustion column appears to be a major factor
oxygen demand (BOD) and chemical oxygen demand (COD)
contributing to interferences of this nature. Similarly, high
analyzers have long time cycles and in the case of COD
concentrationsofdissolvedinorganicsaltswilltendtobuildup
analyzers use corrosive reagents with the inherent problem of
and coat the catalyst as indicated by a loss of sensitivity. To
disposal. Total oxygen demand analysis is faster, approxi-
correct the problem, replace the combustion tube and refrac-
mately 3 min, and uses no liquid reagents in its analysis.
tory packing material and clean the catalyst in accordance with
the manufacturer’s recommendations. The effects of these
5.2 TOD can be correlated to both COD and BOD, provid-
ing effective on-line control. problems can be minimized by dilution of the sample.
D6238 − 98 (2017)
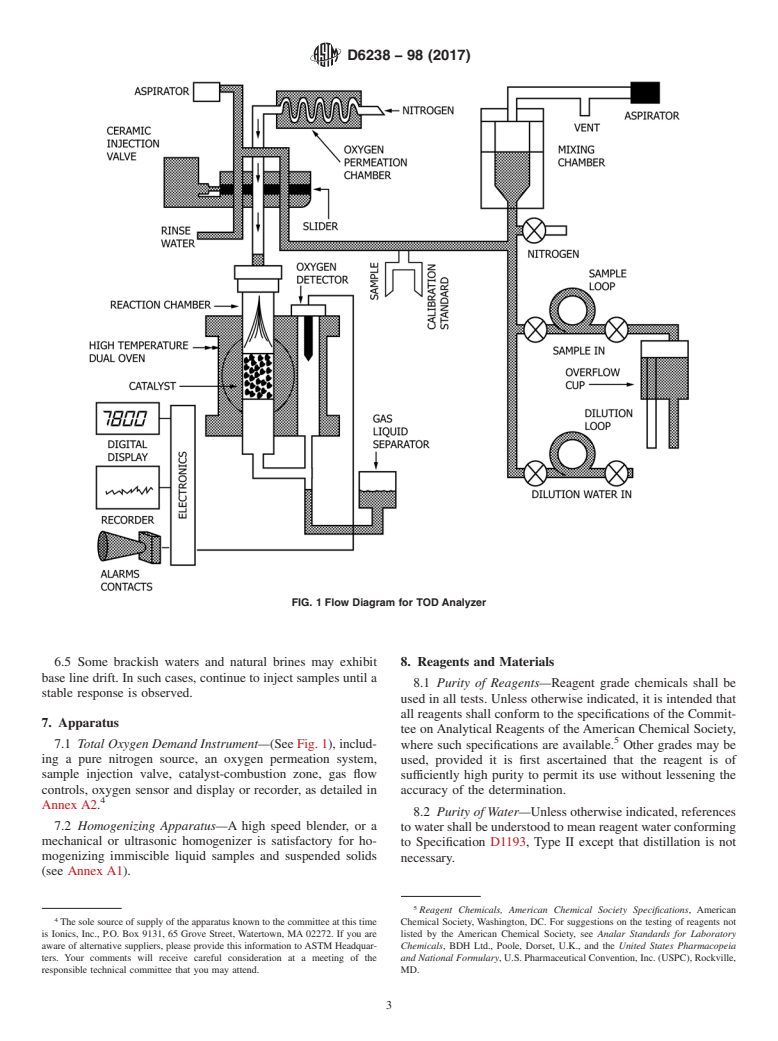
FIG. 1 Flow Diagram for TOD Analyzer
6.5 Some brackish waters and natural brines may exhibit 8. Reagents and Materials
base line drift. In such cases, continue to inject samples until a
8.1 Purity of Reagents—Reagent grade chemicals shall be
stable response is observed.
used in all tests. Unless otherwise indicated, it is intended that
all reagents shall conform to the specifications of the Commit-
7. Apparatus
tee onAnalytical Reagents of theAmerican Chemical Society,
7.1 Total Oxygen Demand Instrument—(See Fig. 1), includ-
where such specifications are available. Other grades may be
ing a pure nitrogen source, an oxygen permeation system,
used, provided it is first ascertained that the reagent is of
sample injection valve, catalyst-combustion zone, gas flow sufficiently high purity to permit its use without lessening the
controls, oxygen sensor and display or recorder, as detailed in
accuracy of the determination.
Annex A2.
8.2 Purity of Water—Unless otherwise indicated, references
7.2 Homogenizing Apparatus—A high speed blender, or a
to water shall be understood to mean reagent water conforming
mechanical or ultrasonic homogenizer is satisfactory for ho-
to Specification D1193, Type II except that distillation is not
mogenizing immiscible liquid samples and suspended solids
necessary.
(see Annex A1).
Reagent Chemicals, American Chemical Society Specifications, American
The sole source of supply of the apparatus known to the committee at this time Chemical Society, Washington, DC. For suggestions on the testing of reagents not
is Ionics, Inc., P.O. Box 9131, 65 Grove Street, Watertown, MA 02272. If you are listed by the American Chemical Society, see Analar Standards for Laboratory
aware of alternative suppliers, please provide this information toASTM Headquar- Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
ters. Your comments will receive careful consideration at a meeting of the and National Formulary, U.S. Pharmaceutical Convention, Inc. (USPC), Rockville,
responsible technical committee that you may attend. MD.
D6238 − 98 (2017)
8.3 Carrier Gas Supply—Prepurified nitrogen containing 10.4 Preliminary Operation:
oxidizable or reducible gases in concentrations of less than 10 10.4.1 Place sample/standard inlet tubing into a full scale
ppmisrecommended.Otherpureinertgases,suchasheliumor standard solution container, and rinse water tubing into a
argon, are acceptable. The required oxygen is added to the deionized water container.
carriergasbymeansofthepermeationsystemintheapparatus. 10.4.2 Place instrument in calibrate, active operation mode.
Alternatively, a bottled, fixed oxygen concentration carrier gas 10.4.3 Operate instrument for several analysis cycles (5 to
may be used in place of a permeation system. 10).
10.4.4 Observe repeatability of analyses to ensure analyzer
8.4 Total Oxygen Demand Calibration Standard Solutions:
is repeating within 63 % of full scale.
8.4.1 Potassium Acid Phthalate (KHP) Solution Stock—
10.4.5 Proceed to calibration and sample analysis.
(10 000 mg/L TOD) Dissolve 8.509 g of potassium acid
phthalate (KHP) in water in a volumetric flask and dilute 1 L.
11. Calibration
This solution is stable for several weeks at average room
11.1 Prepare a series of at least four samples of diluted
temperature but is eventually subject to bacteriological dete-
standard solutions in the desired operating range of the
rioration. Refrigeration extends the shelf-life.
instrument. For example, if the desired full scale range is 5000
8.4.2 AceticAcid Solution, Stock—(111 900mg/LTOD)For
mg/L, prepare dilutions containing 5000, 2500, 1000 and 200
calibration standards above 10 000 mg/L, the use of acetic acid
mg/L of TOD. Pipette 50, 25, 10 and 2.0-mL aliquots of the
is recommended. Pipet 100 mL of glacial acetic acid to a 1-L
10 000-mg⁄L stock standard solution into separate 100-mL
volumetric flask containing approximately 500 mL of water.
volumetric flasks and dilute to volume with reagent water.
Dilute to 1 L with water and mix thoroughly. This solution is
stable for several weeks at average room temperature but is
11.2 In operation, standard (or sample) is drawn from the
eventually subject to bacteriological deterioration. Refrigera- sample/standard inlet tubing to a sample injection valve which
tion extends the shelf-life.
delivers a 20 to 100-µL sample into the combustion chamber.
8.4.3 Water solutions of other pure organic compounds may Insert the sample/standard inlet tubing into the container of
be used as standards based on the compound’s theoretical
standard.
oxygen demand.
NOTE 1—Nominal consumption 10 mL/analysis
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.