ASTM D2760-70(1997)
(Test Method)Standard Test Method for Analysis of Sodium Triphosphate by the Simplified Paper Chromatographic Method (Withdrawn 2000)
Standard Test Method for Analysis of Sodium Triphosphate by the Simplified Paper Chromatographic Method (Withdrawn 2000)
SCOPE
1.1 This method is a single-step method developed to reduce the analysis time for the determination of phosphate distribution in condensed alkali phosphates where a separation of tripoly- and trimetaphosphate is desired.
General Information
Standards Content (Sample)
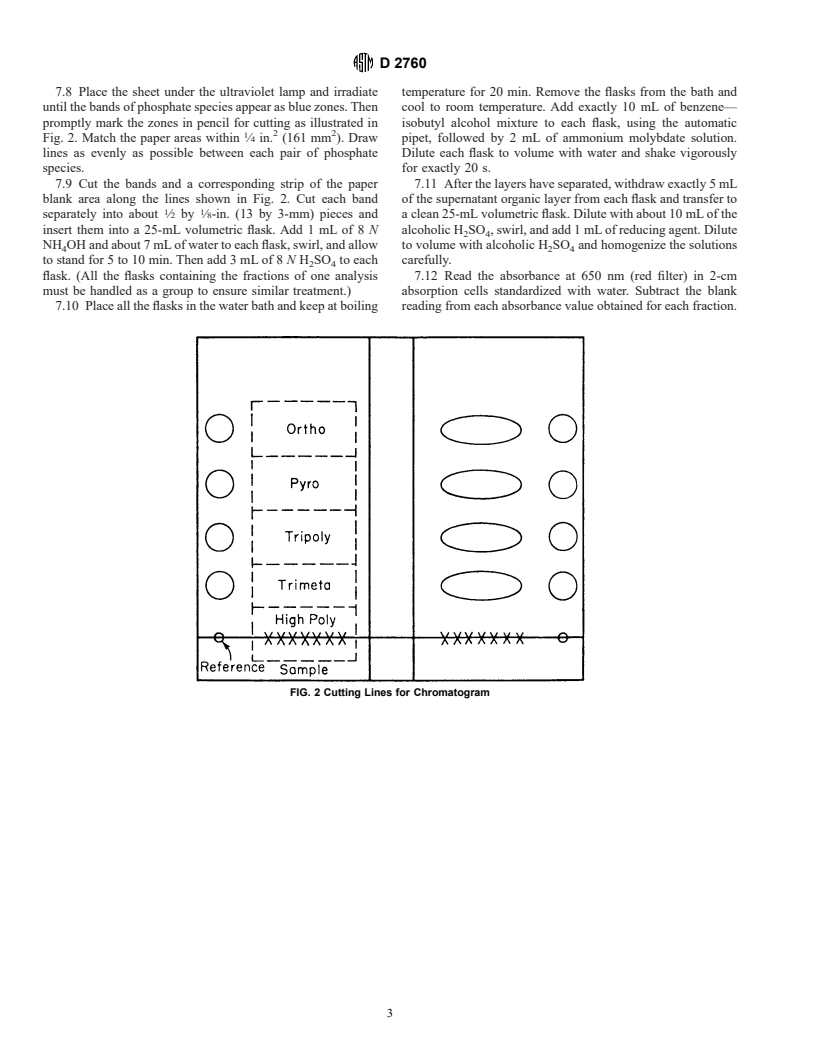
Designation: D 2760 – 70 (Reapproved 1997)
Standard Test Method for
Analysis of Sodium Triphosphate by the Simplified Paper
1
Chromatographic Method
This standard is issued under the fixed designation D 2760; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 4.5 Micropipets, 50-μl capacity, with subdivisions at 10-μl
2
intervals.
1.1 This test method is a single-step method developed to
6
4.6 Pipet Filling Attachment, with screw control.
reduce the analysis time for the determination of phosphate
4.7 Pipets, 5-mL transfer.
distribution in condensed alkali phosphates where a separation
7
4.8 Pipet Filler
of tripoly- and trimetaphosphate is desired.
4.9 Pipet, automatic, 10-mL capacity.
1.2 This standard does not purport to address all of the
4.10 Platinum Wire, about 0.02 in. (0.5 mm) in diameter.
safety concerns, if any, associated with its use. It is the
4.11 Ultraviolet Lamp.
responsibility of the user of this standard to establish appro-
4.12 Water Bath, consisting of a hot plate with several
priate safety and health practices and determine the applica-
600-mL beakers.
bility of regulatory limitations prior to use.
4.13 Refrigerator, maintained at 10°C.
2. Referenced Documents
5. Reagents
2.1 ASTM Standards:
3
5.1 Purity of Reagents—Reagent grade chemicals shall be
D 1193 Specification for Reagent Water
used in all tests. Unless otherwise indicated, it is intended that
3. Summary of Test Method
all reagents shall conform to the specifications of the Commit-
tee on Analytical Reagents of the American Chemical Society,
3.1 Separation of the various phosphate species in con-
8
where such specifications are available. Other grades may be
densed alkali phosphates is accomplished by ascending paper
used, provided it is first ascertained that the reagent is of
chromatography. The individual phosphate species are clearly
sufficiently high purity to permit its use without lessening the
separated in 60 to 75 min. The phosphorus in each of the
accuracy of the determination.
separated bands is determined colorimetrically, and the phos-
5.2 Purity of Water—Unless otherwise indicated, references
phate ion distribution is calculated.
to water shall be understood to mean reagent water conforming
4. Apparatus
to Specification D 1193.
5.3 Ammonium Hydroxide (8 N)—Mix equal volumes of
4.1 Battery Jar, cylindrical, 8 in. (203 mm) in diameter by
concentrated ammonium hydroxide (NH OH, sp gr 0.90) and
10 in. (254 mm) in height, with 10-in. diameter plate-glass 4
water. Adjust the concentration to 8.0 6 0.5 N.
cover.
5.4 Ammonium Molybdate Solution—Dissolve 50 g of am-
4.2 Chromatographic Spray Bottle, 50-mL size.
4
monium molybdate ((NH ) ·Mo O ·4H O) in 450 mL of
4.3 Spectrophotometer 4 6 7 24 2
5
water.
4.4 Filter Paper, special for paper chromatography, in9by
5.5 Chromatographic Solvent—Dissolve 25 g of trichloro-
8-in. (229 by 203-mm) sheets. The necessary markings for
acetic acid in water, add 1.75 mL of concentrated ammonium
these sheets are shown in Fig. 1.
hydroxide (NH OH, sp gr 0.90) and dilute with water to 175
4
mL. Add this to 325 mL of acetone and mix. This solvent can
1
This method is under the jurisdiction of ASTM Committee D-12 on Soaps and
be used for four papers only. Make fresh daily.
Other Detergents and is the direct responsibility of Subcommittee D12.14 on
Analysis of Inorganic Alkaline Detergents.
Current edition approved May 29, 1970. Published July 1970. Originally
6
published as D 2760 – 68. Last previous edition D 2760 – 68 T. A Scientific Glass catalog No. P-6628 attachment has been found satisfactory
2
Based upon Karl-Kroupa, Editha, “Use of Paper Chromatography for Differ- for this purpose.
7
ential Analysis of Phosphate Mixtures,” Analytical Chemistry, Vol 28, 1956, p. A Will Corp. catalog No. 22101 pipet filler has been found satisfactory for this
1091; and Bernhard, D. N., and Chess, W. B., “Quantitative Evaluation of Paper purpose.
8
Reagent Chemicals, American Chemical Society Specifications, American
Chromatograms of Condensed Phosphate Mixtures,” Analytical Chemistry, Vol 31,
1959, p. 1026. Chemical Society, Washington, DC. For suggestions on the testing of reagents not
3
Annual Book of ASTM Standards, Vol 11.01. listed by the American Chemical Society, see Analar Standards for Laboratory
4
A Beckman DU spectrophotometer has been found satisfactory for this purpose. Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
5
Schleicher & Schuell No. 589. Orange Ribbon filter paper has been found and National Formulary, U.S. Pharmacopeial Convention, Inc. (USPC), Rockville,
satisfactory for this purpose. MD.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
1
----------------------
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.