ASTM E928-08(2014)
(Test Method)Standard Test Method for Purity by Differential Scanning Calorimetry
Standard Test Method for Purity by Differential Scanning Calorimetry
SIGNIFICANCE AND USE
5.1 The melting temperature range of a compound broadens as the impurity level rises. This phenomenon is described approximately by the van’t Hoff equation for melting point depressions. Measuring and recording the instantaneous heat flow into the specimen as a function of temperature during such a melting process is a practical way for the generation of data suitable for analysis by the van’t Hoff equation.
5.2 The results obtained include: sample purity (expressed as mole percent); enthalpy of fusion (expressed as joules per mole); and the melting temperature (expressed in Kelvin) of the pure form of the major component.
5.3 Generally, the repeatability of this test method decreases as the purity level decreases. This test method is ordinarily considered unreliable when the purity level of the major component of the mixture is less than 98.5 mol % or when the incremental enthalpy correction (c) exceeds 20 % of the original detected enthalpy of fusion.
5.4 This test method is used for quality control, specification acceptance, and research.
SCOPE
1.1 This test method describes the determination of purity of materials greater than 98.5 mole percent purity using differential scanning calorimetry and the van’t Hoff equation.
1.2 This test method is applicable to thermally stable compounds with well-defined melting temperatures.
1.3 Determination of purity by this test method is only applicable when the impurity dissolves in the melt and is insoluble in the crystal.
1.4 There is no ISO method equivalent to this test method.
1.5 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E928 − 08 (Reapproved 2014)
Standard Test Method for
Purity by Differential Scanning Calorimetry
This standard is issued under the fixed designation E928; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope E1970PracticeforStatisticalTreatmentofThermoanalytical
Data
1.1 Thistestmethoddescribesthedeterminationofpurityof
materials greater than 98.5 mole percent purity using differen-
3. Terminology
tial scanning calorimetry and the van’t Hoff equation.
3.1 Definitions—The definitions relating to thermal analysis
1.2 This test method is applicable to thermally stable
appearing in Terminology E473 shall be considered applicable
compounds with well-defined melting temperatures.
to this test method.
1.3 Determination of purity by this test method is only
4. Summary of Test Method
applicable when the impurity dissolves in the melt and is
insoluble in the crystal.
4.1 Thistestmethodisbaseduponthevan’tHoffequation:
1.4 There is no ISO method equivalent to this test method. 2
T 5 T 2 ~RT χ!/~HF! (1)
s o o
1.5 The values stated in SI units are to be regarded as
where:
standard. No other units of measurement are included in this
T = specimen temperature, K
s
standard.
T = melting temperature of 100% pure material, K
o
−1 −1
1.6 This standard does not purport to address all of the
R = gas constant (= 8.314 J mol K ),
safety concerns, if any, associated with its use. It is the χ = mole fraction of impurity,
−1
responsibility of the user of this standard to establish appro- H = heat of fusion, J mol , and
F = fraction melted.
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use.
4.2 This test method consists of melting the test specimen
that is subjected to a temperature-controlled program while
2. Referenced Documents
recording the heat flow into the specimen as a function of
2.1 ASTM Standards:
temperature.Theresultingmeltingendothermareaismeasured
E473Terminology Relating to Thermal Analysis and Rhe- to yield the enthalpy of fusion, H.The melting endotherm area
ology
is then partitioned into a series of fractional areas (about ten,
E793Test Method for Enthalpies of Fusion and Crystalliza- comprisingthefirst10to50%ofthetotalarea).Thefractional
tion by Differential Scanning Calorimetry
area, divided by the total area, yields the fraction melted, F.
E794TestMethodforMeltingAndCrystallizationTempera- Each fractional area is assigned a temperature, T .
s
tures By Thermal Analysis
4.3 Eq1hastheformofY=mX+bwhereY= T,X=1/F,
s
E967Test Method for Temperature Calibration of Differen- 2
m=−(RT χ)/ H, and b = T . A plot of Y versus X should
o o
tial Scanning Calorimeters and Differential ThermalAna-
produce a straight line with slope m and intercept b.
lyzers
4.4 In practice, however, the resultant plot of T versus 1 /F
s
E968Practice for Heat Flow Calibration of Differential
is seldom a straight line. To linearize the plot, an incremental
Scanning Calorimeters
amount of area is added to the total area and to each fractional
area to produce a revised value for F. The process of
incremental addition of area is continued until a straight line is
ThistestmethodisunderthejurisdictionofASTMCommitteeE37onThermal
Measurements and is the direct responsibility of Subcommittee E37.01 on Calo- obtained.
rimetry and Mass Loss.
F 5 ~A 1c!/~A 1c! (2)
part total
Current edition approved Aug. 15, 2014. Published September 2014. Originally
approved in 1983. Last previous edition approved in 2008 as E928–08. DOI:
10.1520/E0928-08R14.
2 3
For referenced ASTM standards, visit the ASTM website, www.astm.org, or Brennan, W. P., DiVito, M. P., Fynas, R. L., Gray,A. P., “An Overview of the
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Calorimetric Purity Measurement”, in Purity Determinations by Thermal Methods,
Standards volume information, refer to the standard’s Document Summary page on R. L. Blaine and C. K. Schoff (Eds.), Special Technical Publication 838,American
the ASTM website. Society for Testing and Materials, West Conshohocken, PA, 1984, pp. 5–15.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E928 − 08 (2014)
where: 6.2.2 Thesolubilityoftheimpurityinthesolidofthemajor
constituent is negligible; and
A = area of fraction melted, mJ
part
6.2.3 The major constituent displays a single well-defined
A = total area, mJ and
total
c = incremental area, mJ. meltingendotherminthetemperaturerangeofinterest.Micro-
NOTE 1—The best fit straight line may be determined by the least scopic investigations of the melt and the solid may help to
squares method. See Practice E1970.)
establish whether or not solid or liquid solutions have been
formed.
4.5 The values of mole fraction impurity χ and melting
temperatureofthe100%purematerialT aredeterminedfrom 6.2.4 The solute and solvent are close in molecular size.
o
the slope m and intercept b of the resultant straight line. This
6.3 In some cases the sample may react with air during the
is Method A.
temperature cycle, causing an incorrect transition to be mea-
sured. Where it has been shown that this effect is present,
4.6 An alternative form of the van’t Hoff equation is given
by: provision shall be made for sealing the specimen and running
the test under an inert gas blanket. Since some materials
A 52c1 T c 2RT χ m/M /T 1T A /T (3)
@ #
part o o s o part s
degrade near the melting region, carefully distinguish between
where:
degradation and transition. See Appendix X1.
m = mass of the sample, mg, and
6.4 Since milligram quantities of sample are used, ensure
−1
M = molecular weight, g mol .
that samples are homogeneous and representative.
4.7 Eq 3 has the form of Y = αW+ βX+ γ Z where Y =
6.5 Sublimation or decomposition will lead to a different
A , α=−c,W=1, β=[Tc−RT χm/M],X=1/ T , γ
part o o s
heatconsumptionand,perhaps,achangeincompositionofthe
= T ,andZ=A / T . Eq 3 may be evaluated by multiple
o part s
specimen. The specimen holder should be examined after the
linear regression and χ and T determined form the resultant
o
measurement for crystals not part of the resolidified melt.
values of α, β and γ. This is Method B.
7. Apparatus
5. Significance and Use
7.1 The essential equipment required to provide the mini-
5.1 Themeltingtemperaturerangeofacompoundbroadens
mum instrument capability for this test method includes:
as the impurity level rises. This phenomenon is described
7.1.1 Differential Scanning Calorimeter (DSC), consisting
approximately by the van’t Hoff equation for melting point
of:
depressions. Measuring and recording the instantaneous heat
7.1.1.1 DSC Test Chamber, composed of a furnace(s) to
flowintothespecimenasafunctionoftemperatureduringsuch
provide uniform controlled heating of a specimen and refer-
a melting process is a practical way for the generation of data
ence to a constant temperature or at a constant rate within the
suitable for analysis by the van’t Hoff equation.
applicabletemperaturerangeofthistestmethod;atemperature
sensor to provide an indication of the specimen temperature to
5.2 The results obtained include: sample purity (expressed
60.1 K; a differential sensor to detect a heat flow difference
as mole percent); enthalpy of fusion (expressed as joules per
between the specimen and reference equivalent to 10 µW; and
mole); and the melting temperature (expressed in Kelvin) of
a means of sustaining a test chamber environment of N at a
the pure form of the major component.
purge rate of 15 to 50 6 5 mL/min.
5.3 Generally,therepeatabilityofthistestmethoddecreases
7.1.1.2 Temperature Controller, capable of executing a spe-
as the purity level decreases. This test method is ordinarily
cific temperature program by operating the furnace(s) between
considered unreliable when the purity level of the major
selected temperature limits at a rate of temperature change of
component of the mixture is less than 98.5 mol% or when the
0.3 to 0.7 K/min constant to 60.01 K/min.
incremental enthalpy correction (c) exceeds 20% of the
7.1.1.3 Data Collection Device, to provide a means of
original detected enthalpy of fusion.
acquiring, storing, and displaying measured or calculated
5.4 This test method is used for quality control, specifica-
signals,orboth.TheminimumoutputsignalsrequiredforDSC
tion acceptance, and research.
are heat flow, temperature, and time.
7.1.2 Containers,thatareinerttothespecimen,andthatare
6. Interferences
ofsuitablestructuralshapeandintegrityforuseintheDSCtest
6.1 This test method is nonspecific. Many impurities may
chamber, made of materials of high thermal conductivity, such
causethemeltingtemperaturebroadening.Thus,itisnotuseful as aluminum.
in identifying the nature of the impurity or impurities but only
7.2 Planimeter, computer- or electronic-based data treat-
the total mol percent of impurity present.
ment or other instrumentation to determine area to within
6.2 The van’t Hoff theory assumes the following:
61% precision.
6.2.1 The impurities dissolve in the melt of the major
7.3 Balance, with a capacity of at least 100 mg capable of
constituent forming a solution approximately described by
weighing to an accuracy of 0.01 mg.
ideal solution theory;
8. Sampling
8.1 The test sample (liquid or solid) should be mixed prior
Widman,G.,Scherrer,O.,“ANewProgramforDSCPurityAnalysis”, Journal
of Thermal Analysis, 371987, pp. 1957–1964. to sampling and sampled by removing portions from various
E928 − 08 (2014)
parts of the container. Combine the portions and mix well to 10.4 Weigh 1 to 3 mg of the sample to an accuracy of 0.01
provide a representative sample for the purity determinations. mg in a pre-cleaned specimen container.
Only 1 to 3 mg is required for each analysis.
10.5 Under ambient conditions, hermetically seal the speci-
8.2 Avoid any physical or mechanical treatment of the men container so there will be no mass loss during the scan.
material that will cause chemical changes. For example, Minimize the free space between the specimen and the lid to
grinding the sample for size reduction often introduces such avoid sublimation onto the lid.
changes as a result o
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: E928 − 08 E928 − 08 (Reapproved 2014)
Standard Test Method for
Purity by Differential Scanning Calorimetry
This standard is issued under the fixed designation E928; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method describes the determination of purity of materials greater than 98.5 mole percent purity using differential
scanning calorimetry and the van’tvan’t Hoff equation.
1.2 This test method is applicable to thermally stable compounds with well-defined melting temperatures.
1.3 Determination of purity by this test method is only applicable when the impurity dissolves in the melt and is insoluble in
the crystal.
1.4 There is no ISO method equivalent to this test method.
1.5 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
1.6 There is no ISO method equivalent to this test method.
2. Referenced Documents
2.1 ASTM Standards:
E473 Terminology Relating to Thermal Analysis and Rheology
E793 Test Method for Enthalpies of Fusion and Crystallization by Differential Scanning Calorimetry
E794 Test Method for Melting And Crystallization Temperatures By Thermal Analysis
E967 Test Method for Temperature Calibration of Differential Scanning Calorimeters and Differential Thermal Analyzers
E968 Practice for Heat Flow Calibration of Differential Scanning Calorimeters
E1970 Practice for Statistical Treatment of Thermoanalytical Data
3. Terminology
3.1 Definitions—The definitions relating to thermal analysis appearing in Terminology E473 shall be considered applicable to
this test method.
4. Summary of Test Method
4.1 This test method is based upon the van’t Hoff equationequation: :
T 5 T 2 ~RT χ!/~H F! (1)
s o o
where:
T = specimen temperature, K
s
T = melting temperature of 100 % pure material, K
o
This test method is under the jurisdiction of ASTM Committee E37 on Thermal Measurements and is the direct responsibility of Subcommittee E37.01 on Calorimetry
and Mass Loss.
Current edition approved Sept. 1, 2008Aug. 15, 2014. Published November 2008 September 2014. Originally approved in 1983. Last previous edition approved in
20032008 as E928 – 03.E928 – 08. DOI: 10.1520/E0928-08.10.1520/E0928-08R14.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Brennan, W. P., DiVito, M. P., Fynas, R. L., Gray, A. P., “An Overview of the Calorimetric Purity Measurement”, in Purity Determinations by Thermal Methods, R. L.
Blaine and C. K. Schoff (Eds.), Special Technical Publication 838, American Society for Testing and Materials, West Conshohocken, PA 1984, pp. 5 - 15.Brennan, W. P.,
DiVito, M. P., Fynas, R. L., Gray, A. P., “An Overview of the Calorimetric Purity Measurement”, in Purity Determinations by Thermal Methods, R. L. Blaine and C. K. Schoff
(Eds.), Special Technical Publication 838, American Society for Testing and Materials, West Conshohocken, PA, 1984, pp. 5–15.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E928 − 08 (2014)
−1 − 1
R = gas constant (= 8.314 J mol K ),
χ = mole fraction of impurity,
− 1
H = heat of fusion, J mol , and
F = fraction melted.
4.2 This test method consists of melting the test specimen that is subjected to a temperature-controlled program while recording
the heat flow into the specimen as a function of temperature. The resulting melting endotherm area is measured to yield the
enthalpy of fusion, H. The melting endotherm area is then partitioned into a series of fractional areas (about ten, comprising the
first 10 to 50 % of the total area). The fractional area, divided by the total area, yields the fraction melted, F. Each fractional area
is assigned a temperature, T .
s
4.3 Eq 1 has the form of Y = mX +b where Y = T , X = 1/F, m = −(R T χ) / H, and b = T . A plot of Y versus X should produce
s o o
a straight line with slope m and intercept b.
4.4 In practice, however, the resultant plot of T versus 1 /F is seldom a straight line. To linearize the plot, an incremental amount
s
of area is added to the total area and to each fractional area to produce a revised value for F. The process of incremental addition
of area is continued until a straight line is obtained.
F 5 ~A 1c!/~A 1c! (2)
part total
where:
A = area of fraction melted, mJ
part
A = total area, mJ and
total
c = incremental area, mJ.
NOTE 1—The best fit straight line may be determined by the least squares method. See Practice E1970.)
4.5 The values of mole fraction impurity χ and melting temperature of the 100 % pure material T are determined from the slope
o
m and intercept b of the resultant straight line. This is Method A.
4.6 An alternative form of the van’t Hoff equation is given byby: :
A 52c1 T c 2 R T χ m/M /T 1T A /T (3)
@ #
part o o s o part s
where:
m = mass of the sample, mg, and
−1
M = molecular weight, g mol .
4.7 Eq 3 has the form of Y = α W + β X + γ Z where Y = A , α = −c, W = 1, β = [T c − R T χ m / M], X = 1 / T , γ =
part o o s
T ,and Z = A / T .Eq 3 may be evaluated by multiple linear regression and χ and T determined form the resultant values of
o part s o
α, β and γ. This is Method B.
5. Significance and Use
5.1 The melting temperature range of a compound broadens as the impurity level rises. This phenomenon is described
approximately by the van’tvan’t Hoff equation for melting point depressions. Measuring and recording the instantaneous heat flow
into the specimen as a function of temperature during such a melting process is a practical way for the generation of data suitable
for analysis by the van’tvan’t Hoff equation.
5.2 The results obtained include: sample purity (expressed as mole percent); enthalpy of fusion (expressed as joules per mole);
and the melting temperature (expressed in Kelvin) of the pure form of the major component.
5.3 Generally, the repeatability of this test method decreases as the purity level decreases. This test method is ordinarily
considered unreliable when the purity level of the major component of the mixture is less than 98.5 mol % or when the incremental
enthalpy correction (c) exceeds 20 % of the original detected enthalpy of fusion.
5.4 This test method is used for quality control, specification acceptance, and research.
6. Interferences
6.1 This test method is nonspecific. Many impurities may cause the melting temperature broadening. Thus, it is not useful in
identifying the nature of the impurity or impurities but only the total mol percent of impurity present.
6.2 The van’tvan’t Hoff theory assumes the following:
6.2.1 The impurities dissolve in the melt of the major constituent forming a solution approximately described by ideal solution
theory;
6.2.2 The solubility of the impurity in the solid of the major constituent is negligible; and
Widman, G., Scherrer, O., “A New Program for DSC Purity Analysis”, Journal of Thermal Analysis, 371987, pp. 1957–1964.
E928 − 08 (2014)
6.2.3 The major constituent displays a single well-defined melting endotherm in the temperature range of interest. Microscopic
investigations of the melt and the solid may help to establish whether or not solid or liquid solutions have been formed.
6.2.4 The solute and solvent are close in molecular size.
6.3 In some cases the sample may react with air during the temperature cycle, causing an incorrect transition to be measured.
Where it has been shown that this effect is present, provision shall be made for sealing the specimen and running the test under
an inert gas blanket. Since some materials degrade near the melting region, carefully distinguish between degradation and
transition. See Appendix X1.
6.4 Since milligram quantities of sample are used, ensure that samples are homogeneous and representative.
6.5 Sublimation or decomposition will lead to a different heat consumption and, perhaps, a change in composition of the
specimen. The specimen holder should be examined after the measurement for crystals not part of the resolidified melt.
7. Apparatus
7.1 The essential equipment required to provide the minimum instrument capability for this test method includes:
7.1.1 Differential Scanning Calorimeter (DSC), consisting of:
7.1.1.1 DSC Test Chamber, composed of a furnace(s) to provide uniform controlled heating of a specimen and reference to a
constant temperature or at a constant rate within the applicable temperature range of this test method; a temperature sensor to
provide an indication of the specimen temperature to 60.1 K; a differential sensor to detect a heat flow difference between the
specimen and reference equivalent to 10 μW; and a means of sustaining a test chamber environment of N at a purge rate of 15
to 50 6 -55 mL/min.
7.1.1.2 Temperature Controller, capable of executing a specific temperature program by operating the furnace(s) between
selected temperature limits at a rate of temperature change of 0.3 to 0.7 K/min constant to 60.01 K/min.
7.1.1.3 Data Collection Device, to provide a means of acquiring, storing, and displaying measured or calculated signals, or both.
The minimum output signals required for DSC are heat flow, temperature, and time.
7.1.2 Containers, that are inert to the specimen, and that are of suitable structural shape and integrity for use in the DSC test
chamber, made of materials of high thermal conductivity, such as aluminum.
7.2 Planimeter, computer- or electronic-based data treatment or other instrumentation to determine area to within 6 1 % 61 %
precision.
7.3 Balance, with a capacity of at least 100 mg capable of weighing to an accuracy of 0.01 mg.
8. Sampling
8.1 The test sample (liquid or solid) should be mixed prior to sampling and sampled by removing portions from various parts
of the container. Combine the portions and mix well to provide a representative sample for the purity determinations. Only 1 to
3 mg is required for each analysis.
8.2 Avoid any physical or mechanical treatment of the material that will cause chemical changes. For example, grinding the
sample for size reduction often introduces such changes as a result of heat generated by friction.
9. Calibration
9.1 Perform any calibrations procedures called for by the instrument manufacturer as described in the operations manual.
9.2 Calibrate the apparatus temperature signal at the heating rate to be used in this test method (see 10.8) using Test Method
E967. High purity (>99.99 %) indium metal is a convenient material to use for this purpose.
9.3 Calibrate the apparatus heat flow signal at the heating rate to be used in this test method (see 10.8) using Practice E968.
High purity (>99.99 %) indium metal is a convenient material to use for this purpose.
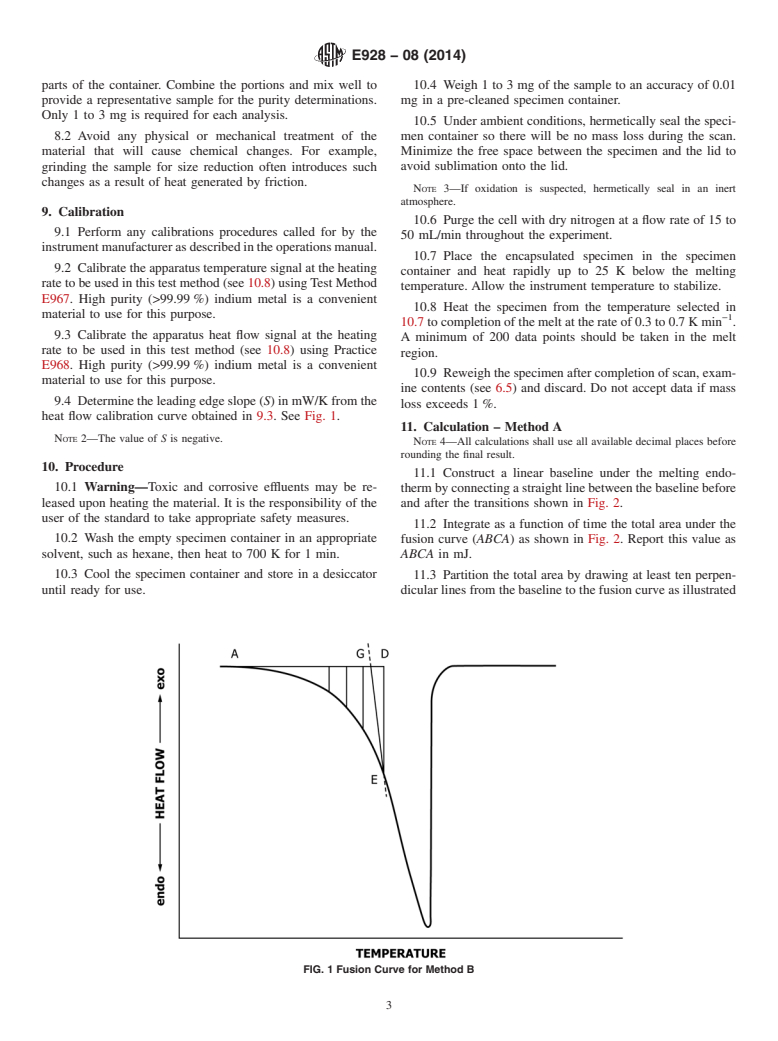
9.4 Determine the leading edge slope (S) in mW/K from the heat flow calibration curve obtained in Section 9.3. See Fig. 1.
NOTE 2—The value of S is negative.
10. Procedure
10.1 Warning—Toxic and corrosive effluents may be released upon heating the material. It is the responsibility of the user of
the standard to take appropriate safety measures.Caution—Toxic and corrosive effluents may be released upon heating the material.
It is the responsibility of the user of the standard to take appropriate safety measures.
10.2 Wash the empty specimen container in an appropriate solvent, such as hexane, then heat to 700 K for 1 min.
10.3 Cool the specimen container and store in a desiccator until ready for use.
10.4 Weigh 1 to 3 mg of the sample to an accuracy of 0.01 mg in a pre-cleaned specimen container.
10.5 Under ambient conditions, hermetically seal the specimen container so there will be no mass loss during the scan.
Minimize the free space between the specimen and the lid to avoid sublimation onto the lid.
E928 − 08 (2014)
FIG. 1 Fusion Curve for Method B
NOTE 3—If oxidation is suspected, hermetically seal in an inert atmosphere.
10.6 Purge the cell with dry nitrogen at a flow rate of 15 to 50 mL/min throughout the experiment.
10.7 Place the encapsulated specimen in the specimen container and heat rapidly up to 25 K below the melting temperature.
Allow the instrument temperature to stabilize.
−1
10.8 Heat the specimen from the temperature selected in 10.7 to completion of the melt at the rate of 0.3 to 0.7 K min . A
minimum of 200 data points should be taken in the melt region.
10.9 Reweigh the specimen after completion of scan, examine contents (see 6.5) and discard. Do not accept data if mass loss
exceeds 1 %.
11. Calculation – Method A
NOTE 4—All calculations shall use all available decimal places before rounding the final result.
11.1 Construct a linear baseline under the melting endotherm by connecting a straight line between the baseline before and after
the transitions shown in Fig. 2.
11.2 Integrate as a function of time the total area under the fusion curve (ABCA) as shown in Fig. 2. Report this value as ABCA
in mJ.
11.3 Partition the total area by drawing at least ten perpendicular lines from the baseline to the fusion curve as illustrated by
the typical line (DE) in Fig. 2. Determine the integrated area of each partial fraction as ADEA in mJ.
11.4 Determine the fraction F for each partial area using Eq 4.
FIG. 2 Schematic of 1/F Plot for Purity Determinations
E928 − 08 (2014)
ADEA
F 5 (4)
ABCA
where:
F = fraction of total area,
ADEA = area of fraction, mJ, and
ABCA = total area under fusion curve, mJ.
11.5 Select at least ten (10) partial area fractions between 10 and 50 % of the total area.
11.6 From the heat flow value (for example DE) calculate the temperature, T , at which each fraction, F, has melted.
F
T 5 T 1DE/S (5)
F D
where:
T = corrected absolute temperature for
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.