ASTM E2041-13e1
(Test Method)Standard Test Method for Estimating Kinetic Parameters by Differential Scanning Calorimeter Using the Borchardt and Daniels Method
Standard Test Method for Estimating Kinetic Parameters by Differential Scanning Calorimeter Using the Borchardt and Daniels Method
SIGNIFICANCE AND USE
6.1 This test method is useful in research and development.
6.2 The determination of the appropriate model for a chemical reaction or transformation and the values associated with its kinetic parameters may be used in the estimation of reaction performance at temperatures or time conditions not easily tested. This use, however, is not described in this test method.
SCOPE
1.1 This test method describes the determination of the kinetic parameters of activation energy, Arrhenius pre-exponential factor, and reaction order using the Borchardt and Daniels2 treatment of data obtained by differential scanning calorimetry. This test method is applicable to the temperature range from 170 to 870 K (−100 to 600 °C).
1.2 This treatment is applicable only to smooth exothermic reactions with no shoulders, discontinuous changes, or shifts in baseline. It is applicable only to reactions with reaction order n ≤ 2. It is not applicable to acceleratory reactions and, therefore, is not applicable to the determination of kinetic parameters for most thermoset curing reactions or to crystallization reactions.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This test method is similar, but not equivalent to, ISO 11357, Part 5, that contains provisions for additional information not supplied by this test method.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
´1
Designation: E2041 − 13
Standard Test Method for
Estimating Kinetic Parameters by Differential Scanning
1
Calorimeter Using the Borchardt and Daniels Method
This standard is issued under the fixed designation E2041; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1
ε NOTE—Warning statements were editorially corrected throughout in September 2013.
1. Scope E473Terminology Relating to Thermal Analysis and Rhe-
ology
1.1 This test method describes the determination of the
E537Test Method for The Thermal Stability of Chemicals
kinetic parameters of activation energy, Arrhenius pre-
by Differential Scanning Calorimetry
exponential factor, and reaction order using the Borchardt and
2 E698Test Method for Arrhenius Kinetic Constants for
Daniels treatment of data obtained by differential scanning
Thermally Unstable Materials Using Differential Scan-
calorimetry. This test method is applicable to the temperature
ning Calorimetry and the Flynn/Wall/Ozawa Method
range from 170 to 870 K (−100 to 600°C).
E967Test Method for Temperature Calibration of Differen-
1.2 This treatment is applicable only to smooth exothermic
tial Scanning Calorimeters and Differential ThermalAna-
reactionswithnoshoulders,discontinuouschanges,orshiftsin
lyzers
baseline. It is applicable only to reactions with reaction order
E968Practice for Heat Flow Calibration of Differential
n ≤ 2. It is not applicable to acceleratory reactions and,
Scanning Calorimeters
therefore, is not applicable to the determination of kinetic
E1142Terminology Relating to Thermophysical Properties
parameters for most thermoset curing reactions or to crystalli-
E1445Terminology Relating to Hazard Potential of Chemi-
zation reactions.
cals
E1641TestMethodforDecompositionKineticsbyThermo-
1.3 The values stated in SI units are to be regarded as
standard. No other units of measurement are included in this gravimetry Using the Ozawa/Flynn/Wall Method
E1970PracticeforStatisticalTreatmentofThermoanalytical
standard.
Data
1.4 This test method is similar, but not equivalent to,
4
2.2 ISO Standards:
ISO11357, Part 5, that contains provisions for additional
ISO11357Part 5: Determination of Temperature and/or
information not supplied by this test method.
Time of Reaction and Reaction Kinetics
1.5 This standard does not purport to address all of the
safety concerns, if any, associated with its use. It is the
3. Terminology
responsibility of the user of this standard to establish appro-
3.1 Definitions—Specific technical terms used in this test
priate safety and health practices and determine the applica-
methodaredefinedinTerminologiesE473,E1142,andE1445,
bility of regulatory limitations prior to use.
including calibration, calorimeter, differential scanning
calorimetry, enthalpy, peak, reaction, repeatability,
2. Referenced Documents
reproducibility, and slope.
3
2.1 ASTM Standards:
4. Summary of Test Method
1
ThistestmethodisunderthejurisdictionofASTMCommitteeE37onThermal 4.1 Atest specimen is heated at a linear rate in a differential
MeasurementsandthedirectresponsibilityofSubcommitteeE37.01onCalorimetry
scanning calorimeter or other suitable calorimeter through a
and Mass Loss.
region of exothermic reaction behavior. The rate of heat
Current edition approved Sept. 15, 2013. Published September 2013. Originally
ε1
evolution, developed by a chemical reaction, is proportional to
approved in 1999. Last previous edition approved in 2008 as E2041–08 . DOI:
10.1520/E2041-13E01.
therateofreaction.Integrationoftheheatflowasafunctionof
2
Borchardt,H.J.,Daniels,F., Journal of theAmerican Chemical Society,Vol79,
time yields the total heat of a reaction.
1957, pp. 41–46.
3
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
4
Standards volume information, refer to the standard’s Document Summary page on Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
the ASTM website. 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
´1
E2041 − 13
2
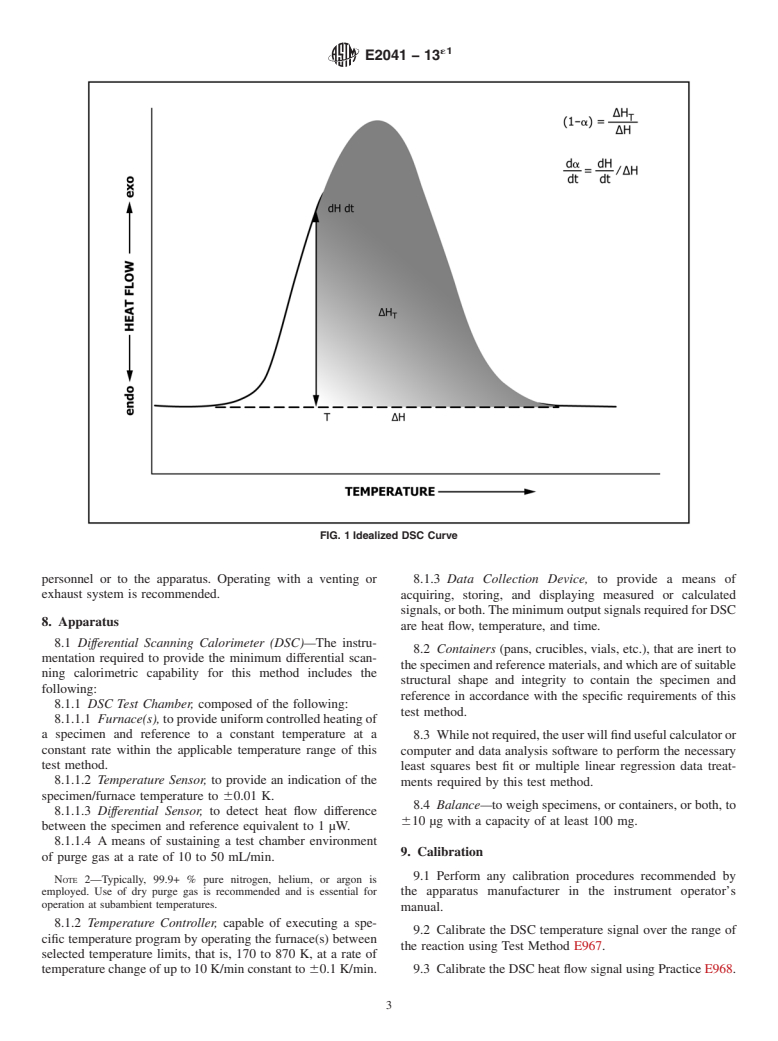
4.2 The Borchardt and Daniels data treatment is used to a single linear heating rate DSC experiment scanning through
derive the kinetic parameters of activation energy, Arrhenius the temperature region of the reaction exotherm as shown in
pre-exponential factor, and reaction order from the heat flow Fig. 1.
and total heat of reaction information obtained in 4.1 (see
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.