ASTM E1558-99(2004)
(Guide)Standard Guide for Electrolytic Polishing of Metallographic Specimens
Standard Guide for Electrolytic Polishing of Metallographic Specimens
SCOPE
1.1 This guide deals with electrolytic polishing as a means of preparation of specimens for metallographic purposes. Procedures are described for polishing a variety of metals.
Note 1—References (1-133) on electrolytic polishing will provide the reader with specific information beyond the scope of this guide.
1.2 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific safety precautions are described in Section 5 and 6.3.1.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:E1558–99 (Reapproved 2004)
Standard Guide for
Electrolytic Polishing of Metallographic Specimens
This standard is issued under the fixed designation E1558; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 4.1.3 There can be a marked saving of time if many
specimens of the same material are polished sequentially.
1.1 This guide deals with electrolytic polishing as a means
4.1.4 Electropolishing a selected area on the surface of a
of preparation of specimens for metallographic purposes.
relatively large metal part can be accomplished nondestruc-
Procedures are described for polishing a variety of metals.
tively, that is, without the need for sectioning to remove a
NOTE 1—References (1-133) onelectrolyticpolishingwillprovidethe
piece.
reader with specific information beyond the scope of this guide.
4.1.5 Soft, single-phase metals, which may be difficult to
1.2 This standard does not purport to address all of the
polishbymechanicalmethods,maybesuccessfullyelectropol-
safety concerns, if any, associated with its use. It is the
ished.
responsibility of the user of this standard to establish appro-
4.1.6 Thetruemicrostructureofaspecimencanbeobtained
priate safety and health practices and determine the applica-
because artifacts (such as disturbed metal, scratches, and
bility of regulatory limitations prior to use. Specific safety
mechanical twins) produced on the surface, even by careful
precautions are described in Section 5 and 6.3.1.
grindingandmechanicalpolishingoperations,canberemoved.
These features are important in low-load hardness testing,
2. Referenced Documents
X-ray diffraction studies, and in electron microscopy, where
2.1 ASTM Standards:
higher resolution puts a premium on undistorted metal sur-
E7 Terminology Relating to Metallography
faces.
E407 Test Methods for Microetching Metals and Alloys
4.1.7 After electropolishing is completed, etching can often
be accomplished by reducing the voltage (generally to about
3. Terminology
one-tenth that required for polishing) for a short time before it
3.1 Definitions—All terms used in this guide are either
is turned off.
defined in Terminology E7 or are discussed in 3.2.
NOTE 2—Not all electropolishing solutions produce good etching
3.2 Definitions of Terms Specific to This Standard:
results.
3.2.1 electrolytic polish (electropolish)—A method of pol-
4.2 Disadvantages of Electrolytic Polishing:
ishingmetalsandalloysinwhichmaterialisremovedfromthe
4.2.1 Many of the chemical mixtures used in electropolish-
surface by making the metal the anode in an electrolytic bath.
ing are poisonous or dangerous if not properly handled (see
4. Significance and Use
Section 5). These hazards are similar to those involved in the
mixing and handling of etchants, see Test Methods E407.
4.1 Advantages of Electrolytic Polishing:
4.2.2 In multi-phase alloys, the polishing rate of each phase
4.1.1 For some metals, a high quality surface finish can be
may be different. The result may be a non-planar surface.
producedthatisequivalentto,orbetterthan,thatwhichcanbe
4.2.3 Electropolished surfaces may be slightly undulated
obtained by mechanical methods.
rather than perfectly planar and, therefore, may not be suitable
4.1.2 Once procedures have been established, satisfactory
for examination at all magnifications.
results can be obtained rapidly with reproducibility.
4.2.4 The rate of polishing in areas adjacent to various
inhomogeneities, such as nonmetallic inclusions and voids, is
ThisguideisunderthejurisdictionofASTMCommitteeE04onMetallography
usuallygreaterthanthatinthesurroundingmatrixandtendsto
and is the direct responsibility of Subcommittee E04.01 on Specimen Preparation.
exaggerate the size of the inclusions and voids.
CurrenteditionapprovedJune1,2004.PublishedJuly2004.Originallyapproved
in 1993. Last previous edition approved in 1999 as E1558–99. 4.2.5 Dimples, pits, and waviness limit applications involv-
The boldface numbers in parentheses refer to the references at the end of this
ingsurfacephenomena,coatings,interfaces,andcracks.Edges
standard.
tend to be attacked preferentially, resulting in edge rounding.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
4.2.6 Artifacts may be produced by electropolishing.
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
E1558–99 (2004)
4.2.7 Specimen mounting materials may react with the read and understood concerning all of the hazards and safety
electrolyte. precautions to be observed. Users should be aware of the type
4.2.8 The electropolished surfaces of certain materials may
of hazards involved in the use of all chemicals used, including
be passive and difficult to etch.
those hazards that are immediate, long-term, visible, invisible,
4.2.9 Metal removal rates by electropolishing are usually
and with or without odors.
quite low, typically about 1 µm/min, and all of the prior
5.1.1 Consult the product labels and MSDS for recommen-
induced damage from cutting and grinding may not be re-
dations concerning proper protective clothing.
moved if preparation is stopped after a 600-grit SiC grind and
5.1.2 All chemicals are potentially dangerous. All persons
electropolishing times are short.
using any electrolyte should be thoroughly familiar with all of
4.2.10 A large number of electrolytes may be needed to
the chemicals involved and the proper procedure for handling,
polish the variety of metals encountered by a given laboratory.
mixing, and disposing of each chemical, as well as any
Considerable time may be required to develop a procedure for
combinations of those chemicals.
a new alloy.
5.1.3 Table 1 includes specific safety precautions for the
5. General Safety Precautions
mixing or use of some electrolytes. The user should take care
5.1 Beforeusingormixinganychemicals,allproductlabels
to observe each of these specific precautions.
and pertinent Material Safety Data Sheets (MSDS) should be
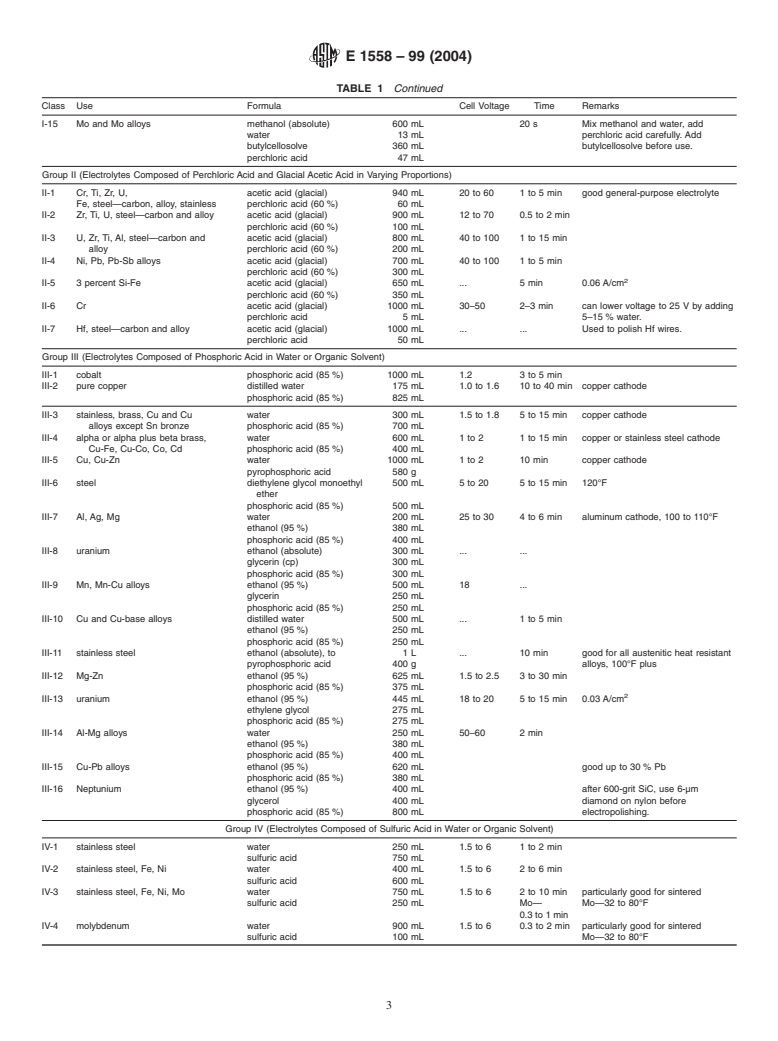
TABLE 1 Electrolytes for Electropolishing
Class Use Formula Cell Voltage Time Remarks
Group I (Electrolytes Composed of Perchloric Acid and Alcohol With or Without Organic Additions)
I-1 Al and Al alloys with less than ethanol (95 %) 800 mL 30 to 80 15 to 60 s
2 percent Si distilled water 140 mL
perchloric acid (60 %) 60 mL
steels—carbon, alloy, stainless 35 to 65 15 to 60 s
Pb, Pb-Sn, Pb-Sn-Cd, Pb-Sn-Sb 12 to 35 15 to 60 s
Zn, Zn-Sn-Fe, Zn-Al-Cu 20 to 60 .
Mg and high Mg alloys . . nickel cathode
I-2 stainless steel and aluminum ethanol (95 %) 800 mL 35 to 80 15 to 60 s
perchloric acid (60 %) 200 mL
I-3 stainless steel ethanol (95 %) 940 mL 30 to 45 15 to 60 s
perchloric acid (65 %) 60 mL
I-4 steel, cast iron, Al, Al alloys, Ni, ethanol (95 %) 700 mL 30 to 65 15 to 60 s one of the best formulas for
Sn, Ag, Be, Ti, Zr, U, 2-butoxy ethanol 100 mL universal use
heat-resisting alloys perchloric acid (30 %) 200 mL
I-5 steels—stainless, alloy, ethanol (95 %) 700 mL 15 to 50 15 to 60 s universal electrolyte comparable to
high-speed; Fe, Al, Zr, Pb glycerin 100 mL I-4
perchloric acid (30 %) 200 mL
I-6 Al, Al-Si alloys ethanol (95 %) 760 mL 35 to 60 15 to 60 s particularly good with Al-Si alloys
diethyl ether 190 mL
perchloric acid (30 %) 50 mL
I-7 Mo, Ti, Zr, U-Zr alloy methanol (absolute) 600 mL 60 to 150 5to30s
2-butoxy ethanol 370 mL
perchloric acid (60 %) 30 mL
I-8 Al-Si alloys methanol (absolute) 840 mL 50 to 100 5to60s
glycerin 125 mL
perchloric acid (65 %) 35 mL
I-9 vanadium methanol (absolute) 590 mL 30 3 s three-second cycles repeated at
2-butoxy ethanol 350 mL least seven times to prevent heating
perchloric acid (65 %) 60 mL
germanium 25 to 35 30 to 60 s
titanium 58 to 66 45 s polish only
zirconium 70 to 75 15 s polish and etch simultaneously
I-10 aluminum methanol (absolute) 950 mL 30 to 60 15 to 60 s
nitric acid 15 mL
perchloric acid (60 %) 50 mL
I-11 steels—carbon, alloy, stainless methanol (absolute) 600 mL 30–40 5–60 s good all purpose electropolish
Ti, high-temperature alloys, Pb, butylcellosolve 360 mL
Mo perchloric acid 60 mL
I-12 Al and Al alloys ethanol (95 %) 1000 mL 10 2 min not good for Al-Cu and Al-Si alloys.
perchloric acid 200 mL Black film forms. Peel off after 1–1.5
min and polish 1 min more.
I-13 steel, Al, Ni, Sn, Ti, Be ethanol (95 %) 700 mL 20 20 s Mix ethanol and water, add
stainless steel butylcellosolve 100 mL perchloric acid carefully. Then, add
Al Ni water 137 mL butylcellosolve before use.
perchloric acid 62 mL
I-14 Ni, Ag, or Cu alloys ethanol (95 %) 700 mL 70–80 15 s
Cd butylcellosolve 100 mL
perchloric acid 200 mL
E1558–99 (2004)
TABLE 1 Continued
Class Use Formula Cell Voltage Time Remarks
I-15 Mo and Mo alloys methanol (absolute) 600 mL 20 s Mix methanol and water, add
water 13 mL perchloric acid carefully. Add
butylcellosolve 360 mL butylcellosolve before use.
perchloric acid 47 mL
Group II (Electrolytes Composed of Perchloric Acid and Glacial Acetic Acid in Varying Proportions)
II-1 Cr, Ti, Zr, U, acetic acid (glacial) 940 mL 20 to 60 1 to 5 min good general-purpose electrolyte
Fe, steel—carbon, alloy, stainless perchloric acid (60 %) 60 mL
II-2 Zr, Ti, U, steel—carbon and alloy acetic acid (glacial) 900 mL 12 to 70 0.5 to 2 min
perchloric acid (60 %) 100 mL
II-3 U, Zr, Ti, Al, steel—carbon and acetic acid (glacial) 800 mL 40 to 100 1to15min
alloy perchloric acid (60 %) 200 mL
II-4 Ni, Pb, Pb-Sb alloys acetic acid (glacial) 700 mL 40 to 100 1 to 5 min
perchloric acid (60 %) 300 mL
II-5 3 percent Si-Fe acetic acid (glacial) 650 mL . 5 min 0.06 A/cm
perchloric acid (60 %) 350 mL
II-6 Cr acetic acid (glacial) 1000 mL 30–50 2–3 min can lower voltage to 25 V by adding
perchloric acid 5mL 5–15 % water.
II-7 Hf, steel—carbon and alloy acetic acid (glacial) 1000 mL . . Used to polish Hf wires.
perchloric acid 50 mL
Group III (Electrolytes Composed of Phosphoric Acid in Water or Organic Solvent)
III-1 cobalt phosphoric acid (85 %) 1000 mL 1.2 3 to 5 min
III-2 pure copper distilled water 175 mL 1.0 to 1.6 10 to 40 min copper cathode
phosphoric acid (85 %) 825 mL
III-3 stainless, brass, Cu and Cu water 300 mL 1.5 to 1.8 5 to 15 min copper cathode
alloys except Sn bronze phosphoric acid (85 %) 700 mL
III-4 alpha or alpha plus beta brass, water 600 mL 1 to 2 1 to 15 min copper or stainless steel cathode
Cu-Fe, Cu-Co, Co, Cd phosphoric acid (85 %) 400 mL
III-5 Cu, Cu-Zn water 1000 mL 1 to 2 10 min copper cathode
pyrophosphoric acid 580 g
III-6 steel diethylene glycol monoethyl 500 mL 5 to 20 5 to 15 min 120°F
ether
phosphoric acid (85 %) 500 mL
III-7 Al, Ag, Mg water 200 mL 25 to 30 4 to 6 min aluminum cathode, 100 to 110°F
ethanol (95 %) 380 mL
phosphoric acid (85 %) 400 mL
III-8 uranium ethanol (absolute) 300 mL . .
glycerin (cp) 300 mL
phosphoric acid (85 %) 300 mL
III-9 Mn, Mn-Cu alloys ethanol (95 %) 500 mL 18 .
glycerin 250 mL
phosphoric acid (85 %) 250 mL
III-10 Cu and Cu-base alloys distilled water 500 mL . 1to5min
ethanol (95 %) 250 mL
phosphoric acid (85 %) 250 mL
III-11 stainless steel ethanol (absolute), to 1L . 10 min good for all austenitic heat resistant
pyrophosphoric acid 400 g alloys, 100°F plus
III-12 Mg-Zn ethanol (95 %) 625 mL 1.5to2.5 3to30min
phosphoric acid (85 %) 375 mL
III-13 uranium ethanol (95 %) 445 mL 18 to 20 5 to 15 min 0.03 A/cm
ethylene glycol 275 mL
phosphoric acid (85 %) 275 mL
III-14 Al-Mg alloys water 250 mL 50–60 2 min
ethanol (95 %) 380 mL
phosphoric acid (85 %) 400 mL
III-15 Cu-Pb alloys ethanol (95 %) 620 mL good up to 30 % Pb
phosphoric acid (85 %) 380 mL
III-16 Neptunium ethanol (95 %) 400 mL after 600-grit SiC, use 6-µm
glycerol 400 mL diamond on nylon before
phosphoric acid (85 %) 800 mL electropolishing.
Group IV (Electrolytes Composed of Sulfuric Acid in Water or Organic Solvent)
IV-1 stainless steel water 250 mL 1.5to6 1to2min
sulfuric acid 750 mL
IV-2 stainless steel, Fe, Ni water 400 mL 1.5to6 2to6min
sulfuric acid 600 mL
IV-3 stainless steel, Fe, Ni, Mo water 750 mL 1.5to6 2to10min particularly good for sintered
sulfuric acid 250 mL Mo— Mo—32 to 80°F
0.3to1min
IV-4 molybdenum water 900 mL 1.5 to 6 0.3 to 2 min particularly good for sintered
sulfuric acid 100 mL Mo—32 to 80°F
E1558–99 (2004)
TABLE 1 Continued
Class Use Formula Cell Voltage Time Remarks
IV-5 stainless steel water 70 mL 1.5to6 0.5to5min
glycerin 200 mL
sulfuric acid 720 mL
IV-6 stainless steel, aluminum water 220 mL 1.5 to 12 1 to 20 min
glycerin 200 mL
sulfuric acid 580 mL
IV-7 molybdenum methanol (absolute) 875 mL 6 to 18 0.5 to 1.5 min 32 to 80°F
sulfuric acid 125 mL
IV-8 Ni-base superalloys methanol (absolute) 800 mL 30 20 s for alloy 625
sulfuric acid 200 mL
Group V (Electrolytes Composed of Chromic Acid in Water)
V-1 stainless steel water 830 mL 1.5to9 2to10min
chromic acid 620 g
V-2 Zn, brass water 830 mL 1.5to12 10to60s
chromic acid 170 g
Group VI (Mixed Acids or Salts in Water or Organic Solvent)
VI-1 stainless steel phosphoric acid (85 %) 600 mL . .
sulfuric acid 400 mL
VI-2 stainless steel water 150 mL . 2 min 0.3 A/cm
phosphoric acid (85 %) 300 mL
sulfuric acid 550 mL
VI-3 stainless and alloy steel water 240 mL . 2to10min 0.1to0.2A/cm
phosphoric acid (85 %) 420 mL
sulfuric acid 340 mL
VI-4 stainless steel water 330 mL . 1 min 0.05 A/cm
phosphoric acid (85 %) 550 mL
sulfuric acid 120 mL
VI-5 bronze (to 9 % Sn) water 450 mL . 1 to 5 min 0.1 A/cm
phosphoric acid (85 %) 390 mL
sulfuric acid 160 mL
VI-6 bronze (to 6 % Sn) water 330 mL . 1 to 5 min 0.1 A/cm
phosphoric acid (85 %) 580 mL
sulfuric acid 90 mL
VI-7 steel water 140 mL . 1to5min 1to5A/cm , 100°F plus
glycerin 100 mL
phosphoric acid (85 %) 430 mL
sulfuric acid 330 mL
VI-8 stainless steel water 200 mL . 5 min 1 A/cm , 80 to 120°F
glycerin 590 mL
phosphoric acid (85 %) 100 mL
sulfuric acid 110 mL
VI-9 stainless steel water 260 mL . 30 min 0.6 A/cm , 80 to 120°F
chromic acid 175 g
phosphoric acid (85 %) 175 mL
sulfuric acid 580 mL
VI-10 stainless steel water 175 mL . 60 min 0.5 A/cm , 80 to 120°F
chromic acid 105 g
phosphoric acid (85 %) 460 mL
sulfuric acid 390 mL
VI-11 stainless and alloy steel water 240 mL . 5to60min 0.5toA/cm , 100 to 130°F
chromic acid 80 g
phosphoric acid (85 %) 650 mL
sulfuric acid 130 mL
VI-12 tanta
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.