ASTM E2070-00
(Test Method)Standard Test Method for Kinetic Parameters by Differential Scanning Calorimetry Using Isothermal Methods
Standard Test Method for Kinetic Parameters by Differential Scanning Calorimetry Using Isothermal Methods
SCOPE
1.1 Test Method A determines kinetic parameters for activation energy, frequency factor and reaction order using differential scanning calorimetry from a series of isothermal experiments over a small ( 10 K) temperature range. This treatment is applicable to low nth order reactions and to autocatalyzed reactions such as thermoset curing or pyrotechnic reactions and crystallization transformations in the temperature range from 300 to 900 K (30 to 630 C). This test method is applicable only to these types of exothermic reactions when the thermal curves do not exhibit shoulders, discontinuities or shifts in baseline.
1.2 Test Method B also determines the activation energy of a set of time-to-event and isothermal temperature data generated by this or other procedures.
1.3 Electronic instrumentation or automated data analysis systems or treatments equivalent to this test method may be used.Note 1
Since all electronic data systems are not equivalent, the user must verify the applicability of the treatment to this method.
1.4 SI units are the standard.
1.5 This test method is similar but not equivalent to ISO 11357, Part 5, and provides more information than the ISO standard.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific precautionary statements are given in Section .
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E 2070 – 00
Standard Test Method for
Kinetic Parameters by Differential Scanning Calorimetry
Using Isothermal Methods
This standard is issued under the fixed designation E 2070; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope D 4565 Test Method for Physical and Environmental Per-
formance Properties of Insulations and Jackets for Tele-
1.1 Test Method A determines kinetic parameters for acti-
communications Wire and Cable
vation energy, frequency factor and reaction order using
D 5483 Test Method for Oxidation Induction Time of Lu-
differential scanning calorimetry from a series of isothermal
bricating Greases by Pressure Differential Scanning Calo-
experiments over a small (; 10 K) temperature range. This
rimetry
treatment is applicable to low nth order reactions and to
D 6186 Test Method for Oxidation Induction Time of Lu-
autocatalyzed reactions such as thermoset curing or pyrotech-
bricating Oils by Pressure Differential Scanning Calorim-
nic reactions and crystallization transformations in the tem-
etry
perature range from 300 to 900 K (30 to 630 °C). This test
E 473 Terminology Relating to Thermal Analysis
method is applicable only to these types of exothermic reac-
E 537 Test Method for Assessing the Thermal Stability of
tions when the thermal curves do not exhibit shoulders,
Chemicals by Method of Differential Thermal Analysis
discontinuities or shifts in baseline.
E 698 Test Method for Arrhenius Kinetic Constants for
1.2 Test Method B also determines the activation energy of
Thermally Unstable Materials
a set of time-to-event and isothermal temperature data gener-
E 967 Test Method for Temperature Calibration of Differ-
ated by this or other procedures.
ential Thermal Analyzers and Differential Scanning Calo-
1.3 Electronic instrumentation or automated data analysis
rimeters
systems or treatments equivalent to this test method may be
E 968 Test Method for Heat Flow Calibration of Differen-
used.
tial Scanning Calorimeters
NOTE 1—Since all electronic data systems are not equivalent, the user
E 1142 Terminology Relating to Thermophysical Proper-
must verify the applicability of the treatment to this method.
ties
1.4 SI units are the standard.
E 1445 Terminology Relating to Hazardous Properties of
1.5 This test method is similar but not equivalent to ISO
Materials
11357, Part 5, and provides more information than the ISO
E 1860 Test Method for Elapsed Time Calibration of Ther-
standard.
mal Analyzers
1.6 This standard does not purport to address all of the
E 1958 Test Method for Oxidative Induction Time of Hy-
safety concerns, if any, associated with its use. It is the
drocarbons by Differential Scanning Calorimetry
responsibility of the user of this standard to establish appro-
E 1970 Practice for Statistical Treatment of Thermoanalyti-
priate safety and health practices and determine the applica-
cal Data
bility of regulatory limitations prior to use. Specific precau-
E 2041 Test Method for Kinetic Parameters by the Bor-
tionary statements are given in Section 8.
chardt and Daniels Method using Differential Scanning
Calorimetry
2. Referenced Documents
E 2046 Test Method for Reaction Induction Time by Ther-
2.1 ASTM Standards:
mal Analysis
D 3350 Specification for Polyethylene Plastic Pipe
2.2 ISO Standard:
D 3895 Test Method for Oxidative Induction Time of Poly-
ISO DIS 11357 Part 5 Determination of Temperature and/or
olefins by Differential Scanning Calorimetry
Time of Reaction and Reaction Kinetics
1 3
This test method is under the jurisdiction of ASTM Committee E-37 on Annual Book of ASTM Standards, Vol 10.02.
Thermal Measurements and is the direct responsibility of Subcommittee E37.01 on Annual Book of ASTM Standards, Vol 05.03.
Thermal Analysis Test Methods. Annual Book of ASTM Standards, Vol 14.02.
Current edition approved March 10, 2000. Published June 2000. Available from American National Standards Institute, 11 W. 42nd St., 13th
Annual Book of ASTM Standards, Vol 08.02. Floor, New York, NY 10036.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
E 2070
3. Terminology This equation has the form z 5 a + bx + cy and may be
solved using multiple linear regression analysis.
3.1 Specific technical terms used in this test method are
defined in Terminologies E 473, E 1142, and E 1445.
NOTE 3—Subsequent discussions use the autocatalytic form of the rate
equation (Eq 3). It reduces to the simpler general rate equation (Eq 2)
4. Summary of Test Method
when the value of reaction order parameter m equals zero thereby reducing
the number of kinetic parameters to be determined.
4.1 A test specimen is held at a constant temperature in a
differential scanning calorimeter throughout an exothermic
5.4 The Arrhenius equation describes how the reaction rate
reaction. The rate of heat evolution, developed by the reaction,
changes as a function of temperature:
is proportional to the rate of reaction. Integration of the heat
2E/RT
k~T! 5 Ze (6)
flow as a function of time yields the total heat of reaction.
, ,
7 8 9
4.2 An autocatalytic or nth order data treatment is used
where:
to derive the kinetic parameters of activation energy, frequency
–1
Z 5 frequency factor (min ),
–1
factor and reaction order from the heat flow and total heat of
E 5 activation energy (J mol ),
reaction information obtained in 4.1 (See Basis for Methodol-
T 5 absolute temperature (K),
–1 –1
ogy, Section 5.)
R 5 gas constant 5 8.314 J mol K ), and
e 5 natural logarithm base 5 2.7182818.
5. Basis of Methodology
5.5 Eq 6 cast in its logarithmic form is:
5.1 Reactions of practical consideration are exothermic in
ln k T! 5 ln Z – E/RT (7)
@ ~ # @ #
nature; that is, they give off heat as the reaction progresses.
Furthermore, the rate of heat evolution is proportional to the
Eq 7 has the form of a straight line, y 5 mx + b, where a plot
rate of the reaction. Differential scanning calorimetry measures of the logarithm of the reaction rate constant (ln[k (T)]) versus
heat flow as a dependent experimental parameter. DSC is the reciprocal of absolute temperature (l/T) is linear with the
useful for the measurement of the total heat of a reaction and slope equal to –E/R and an intercept equal to ln[Z].
the rate of the reaction as a function of time and temperature.
5.6 As an alternative to 5.3 and 5.5, the rate and Arrhenius
5.2 Reactions may be modeled with a number of suitable
equations combined and cast in logarithmic form is:
equations of the form of:
ln@da/dt# 5 ln@Z# – E/RT 1 mln@a# 1 nln@1– a# (8)
da/dt 5 k~T! f~a! (1)
Eq 8 has the form, z 5 a + bw + cx + dy, and may be solved
using multiple linear regression analysis.
where:
–1
da/dt 5 reaction rate (min ), 5.7 If activation energy values only are of interest, Eq 8 may
a5 fraction reaction or conversion (dimensionless),
be solved under conditions of constant conversion to yield:
–1
k (T) 5 specific rate constant at temperature T (min ),
ln Dt 5 E/RT 1 c (9)
@ #
f(a) 5 conversion function. Commonly used functions
include:
where:
n Dt 5 lapsed time at isothermal temperature, T, and
f ~a! 5 ~1– a! (2)
c 5 constant.
m n
f ~a!5a ~1– a! (3)
Eq 9 has the form of a straight line, y 5 mx + b, where a plot
of the logarithm of the lapsed time under a series of differing
where:
isothermal conditions versus the reciprocal of absolute tem-
n and m 5 partial reaction order terms.
perature (l/T) is linear with a slope equal to E/R.
NOTE 2—There are a large number of conversion function expressions
5.8 A series of isothermal experiments by Test Method A
for [f(a)] . Those described here are the more common but are not the
described in Section 11 at four or more temperatures, deter-
only functions suitable for this method. Eq 2 is known as the general rate
8,9
mines the kinetic parameters of activation energy, frequency
equation while Eq 3 is the autocatalytic (or Sestak-Berggren) equation .
factor and reaction order. Alternatively, the time to a condition
Eq 2 is used for nth order reactions while Eq 3 is used for thermoset cure
and crystallization transformations.
of constant conversion for a series of experiments at four or
more temperatures obtained by this or alternative Test Method
5.3 For a reaction conducted at temperature (T), the auto-
B, described in Section 12, may be used to determine activation
catalytic rate equation of 5.2 may be cast in its logarithmic
energy only.
form.
5.9 A series of not less than four isothermal DSC experi-
m n
da/dt 5 k T a 1– a (4)
~ ! ~ !
ments, covering a temperature range of approximately 10 K
ln@da/dt# 5 ln@k~T!# 1 mln@a# 1 nln@1– a# (5)
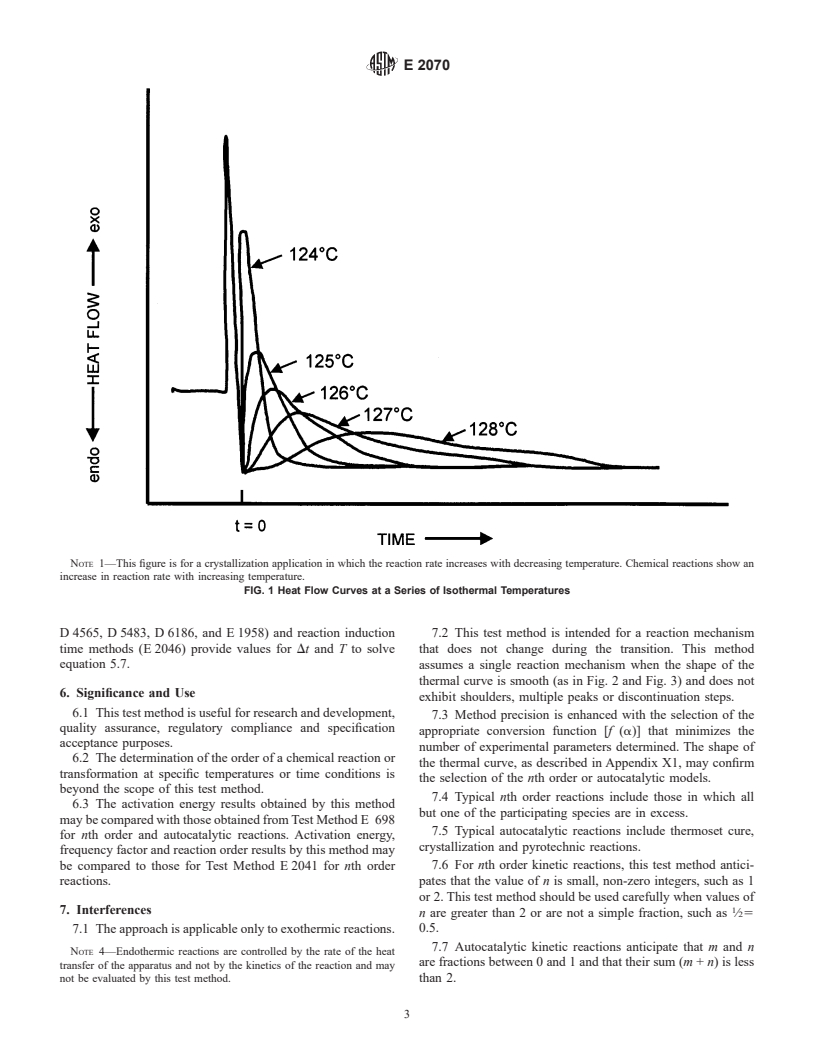
and a time less than 100 min (such as those shown in Fig. 1)
provides values for da/dt, a,(1– a) and T to solve Eq 5, Eq
7, and Eq 8.
Sbirrazzuoli, N.; Brunel, D.; Elegant, L., J. Therm. Anal., 38, 1509-1524, 1992.
8 5.10 A variety of time-to-event experiments such as oxida-
Sestak, J.; Berggren, G.; Thermochim. Acta, 3, 1, 1971.
Gorbachiev, V.M., J. Therm. Anal., 18, 193–197, 1980. tion induction time methods (Test Methods D 3350, D 3895,
E 2070
NOTE 1—This figure is for a crystallization application in which the reaction rate increases with decreasing temperature. Chemical reactions show an
increase in reaction rate with increasing temperature.
FIG. 1 Heat Flow Curves at a Series of Isothermal Temperatures
D 4565, D 5483, D 6186, and E 1958) and reaction induction 7.2 This test method is intended for a reaction mechanism
time methods (E 2046) provide values for Dt and T to solve that does not change during the transition. This method
equation 5.7.
assumes a single reaction mechanism when the shape of the
thermal curve is smooth (as in Fig. 2 and Fig. 3) and does not
6. Significance and Use
exhibit shoulders, multiple peaks or discontinuation steps.
6.1 This test method is useful for research and development,
7.3 Method precision is enhanced with the selection of the
quality assurance, regulatory compliance and specification
appropriate conversion function [f (a)] that minimizes the
acceptance purposes.
number of experimental parameters determined. The shape of
6.2 The determination of the order of a chemical reaction or
the thermal curve, as described in Appendix X1, may confirm
transformation at specific temperatures or time conditions is
the selection of the nth order or autocatalytic models.
beyond the scope of this test method.
7.4 Typical nth order reactions include those in which all
6.3 The activation energy results obtained by this method
but one of the participating species are in excess.
may be compared with those obtained from Test Method E 698
7.5 Typical autocatalytic reactions include thermoset cure,
for nth order and autocatalytic reactions. Activation energy,
crystallization and pyrotechnic reactions.
frequency factor and reaction order results by this method may
be compared to those for Test Method E 2041 for nth order 7.6 For nth order kinetic reactions, this test method antici-
reactions. pates that the value of n is small, non-zero integers, such as 1
or 2. This test method should be used carefully when values of
7. Interferences
n are greater than 2 or are not a simple fraction, such as ⁄25
0.5.
7.1 The approach is applicable only to exothermic reactions.
7.7 Autocatalytic kinetic reactions anticipate that m and n
NOTE 4—Endothermic reactions are controlled by the rate of the heat
are fractions between 0 and 1 and that their sum (m + n) is less
transfer of the apparatus and not by the kinetics of the reaction and may
not be evaluated by this test method. than 2.
E 2070
FIG. 2 Heat Flow Curve for an nth Order Reaction
7.8 Since this method uses milligram quantities it is essen- 9.1.1.4 A means of sustaining a purge gas rate of 10 to 50 6
tial that the test specimens are homogeneous and representative mL/minute in the test chamber.
of the larger samples from which they are taken.
NOTE 5—Typically inert purge gases that inhibit sample oxidation are
7.9 Test specimens may release toxic and corrosive effluents
99.9+ % pure nitrogen, helium or argon. Dry gases are recommended for
that may be harmful to personnel or apparatus. Operation with
all experiments unless the effect of moisture is part of the study.
a venting or exhaust system is recommended.
9.1.2 A Temperature Controller, for furnace(s) temperature
programs between selected temperature limits (that is, 300 to
8. Hazards
900 K) capable of controlling the rate of temperature change of
8.1 Special precautions shall be taken to protect personnel –1 –1
up to 100 K min constant to 6 0.1 K min .
and equipment when the apparatus in use requires the insertion
9.1.3 A Recording Device, digital or analog, capable of
of specimens into a heated furnace. These special precautions
recording and displaying fractions of the heat flow signal (DSC
include adequate shielding and ventilation of equipment and
curve), including the signal noise, on the Y-axis versus frac-
face and hand protections for users (See Note 8.)
tions of time, including the signal noise, on the X-axis.
9.2 Containers (pans, crucibles, vials, etc. and lids) that are
9. Apparatus
inert to the specimen and reference materials of suitable
9.1 A differential scanning calorimeter (DSC) that provides
structural shape and integrity to contain the specimen and
the minimum calorimetric capability for this method includes:
reference in accordance with the requirements of this test
9.1.1 A DSC Test Chamber, composed of:
method.
9.1.1.1 A Furnace(s), that provides uniform controlled heat-
9.3 A Balance, to weigh specimens and/or containers to 6
ing of a specimen and reference to constant temperature at a
10 μg with a capacity of at least 100 mg.
constant rate within the applicable temperature range of this
9.4 Calculation, capability to perform multiple linear re-
test method.
gression analysis for four or more unknowns.
9.1.1.2 A Temperature Sensor, that indicates the specimen/
10. Calibration
furnace temperature to 6 0.01 K.
9.1.1.3 A Differential Sensor, that detects heat flow differ- 10.1 Perform set up and calibration procedures according to
ences between the specimen and reference equivalent to 1 μW. the instrument operator’s manual.
E 2070
FIG. 3 Heat Flow Curve for an Autocatalyzed Reaction
NOTE 7—In some instruments that do not measure the
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.