ASTM F303-08(2023)e1
(Practice)Standard Practices for Sampling for Particles in Aerospace Fluids and Components
Standard Practices for Sampling for Particles in Aerospace Fluids and Components
SIGNIFICANCE AND USE
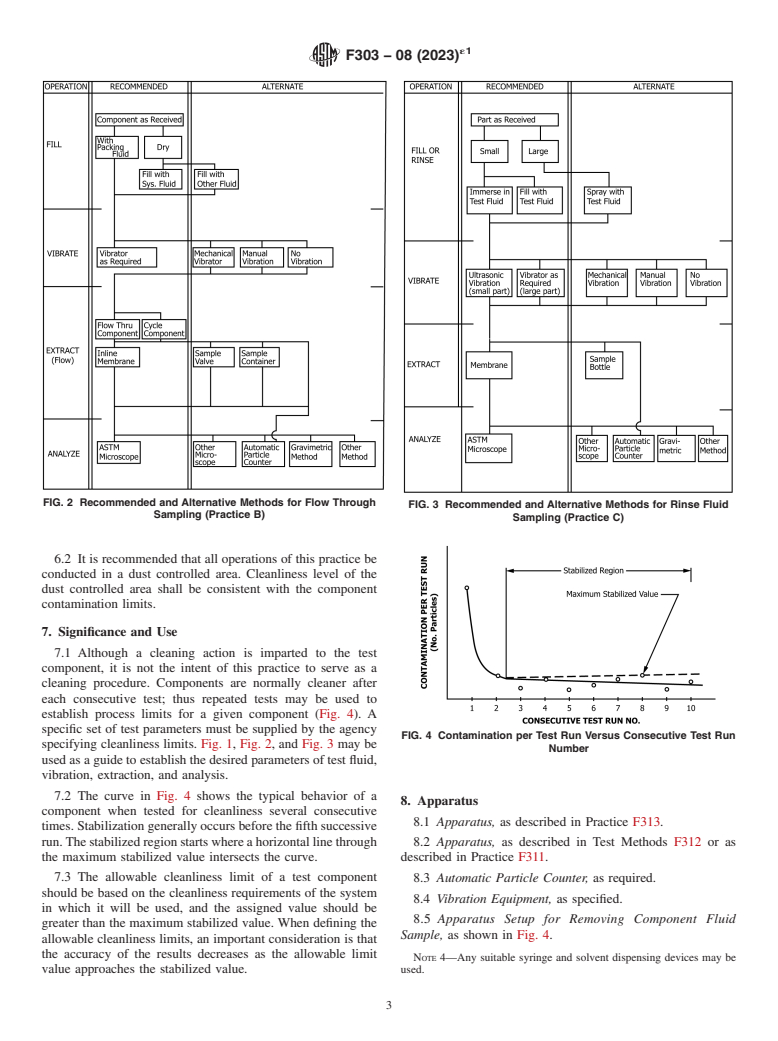
7.1 Although a cleaning action is imparted to the test component, it is not the intent of this practice to serve as a cleaning procedure. Components are normally cleaner after each consecutive test; thus repeated tests may be used to establish process limits for a given component (Fig. 4). A specific set of test parameters must be supplied by the agency specifying cleanliness limits. Fig. 1, Fig. 2, and Fig. 3 may be used as a guide to establish the desired parameters of test fluid, vibration, extraction, and analysis.
FIG. 4 Contamination per Test Run Versus Consecutive Test Run Number
7.2 The curve in Fig. 4 shows the typical behavior of a component when tested for cleanliness several consecutive times. Stabilization generally occurs before the fifth successive run. The stabilized region starts where a horizontal line through the maximum stabilized value intersects the curve.
7.3 The allowable cleanliness limit of a test component should be based on the cleanliness requirements of the system in which it will be used, and the assigned value should be greater than the maximum stabilized value. When defining the allowable cleanliness limits, an important consideration is that the accuracy of the results decreases as the allowable limit value approaches the stabilized value.
SCOPE
1.1 These practices cover sampling procedures for use in determining the particle cleanliness of liquids and liquid samples from components. Three practices, A, B, and C, have been developed on the basis of component geometry in order to encompass the wide variety of configurations. These practices establish guidelines to be used in preparing detailed procedures for sampling specific components.
Note 1: The term cleanliness used in these practices refers to solid particles in the liquid. It does not generally cover other foreign matter such as gases, liquids, and products of chemical degradation. Cleanliness with respect to particulate contamination does not necessarily give any indication of the other types of contamination.
1.2 All components, regardless of application, may be tested provided (1) the fluid medium selected is completely compatible with the materials, packing and fluid used in the test component, and test apparatus, and (2) the fluid is handled in accordance with the manufacturer's recommendations and precautions. A liquid shall be used as the test fluid medium. These test fluids may be flushing, rinsing, packing, end use operating, or suitable substitutes for end use operating fluids. (Warning—Practices for sampling surface cleanliness by the vacuum cleaner technique (used on clean room garments and large storage tanks) sampling gaseous fluids and handling hazardous fluids such as oxidizers, acids, propellants, and so forth, are not within the scope of the practices presented; however, they may be included in addendums or separate practices at a later date.
Substitute fluids are recommended in place of end item fluids for preassembly cleanliness determinations on components using hazardous end item fluids. After obtaining the sample, the substitute fluid must be totally removed from the test part with particular caution given to the possibility of trapped fluid. It is hazardous to use a substitute fluid for testing assembled parts where the fluid can be trapped in dead ends, behind seals, and so forth.)
Note 2: The word fluid used in these practices shall be assumed to be a liquid, unless otherwise stated.
1.3 The cleanliness of assemblies with or without moving parts may be determined at the time of test; however, movement of internal component parts during the test will create unknown quantities of contamination from wear. Practice B covers configurations requiring dynamic actuation to achieve a sample. The practice does not differentiate between built-in particles and wear particles.
Note 3: Defining allowable cleanliness limits is not within the scope of these practice...
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
´1
Designation: F303 − 08 (Reapproved 2023)
Standard Practices for
Sampling for Particles in Aerospace Fluids and
Components
This standard is issued under the fixed designation F303; the number immediately following the designation indicates the year of original
adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A superscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
ε NOTE—Editorially updated formatting in 13.1 and 20.3.3 in September 2023.
1. Scope It is hazardous to use a substitute fluid for testing assembled
parts where the fluid can be trapped in dead ends, behind seals,
1.1 These practices cover sampling procedures for use in
and so forth.)
determining the particle cleanliness of liquids and liquid
samples from components. Three practices, A, B, and C, have
NOTE 2—The word fluid used in these practices shall be assumed to be
been developed on the basis of component geometry in order to
a liquid, unless otherwise stated.
encompass the wide variety of configurations. These practices
1.3 The cleanliness of assemblies with or without moving
establish guidelines to be used in preparing detailed procedures
parts may be determined at the time of test; however, move-
for sampling specific components.
ment of internal component parts during the test will create
NOTE 1—The term cleanliness used in these practices refers to solid
unknown quantities of contamination from wear. Practice B
particles in the liquid. It does not generally cover other foreign matter such
covers configurations requiring dynamic actuation to achieve a
as gases, liquids, and products of chemical degradation. Cleanliness with
sample. The practice does not differentiate between built-in
respect to particulate contamination does not necessarily give any indica-
particles and wear particles.
tion of the other types of contamination.
1.2 All components, regardless of application, may be tested
NOTE 3—Defining allowable cleanliness limits is not within the scope
provided (1) the fluid medium selected is completely compat- of these practices.
ible with the materials, packing and fluid used in the test
1.4 The three practices included are as follows:
component, and test apparatus, and (2) the fluid is handled in
Sections
accordance with the manufacturer’s recommendations and
Practice A—Static Fluid Sampling (Method for 5 – 13
precautions. A liquid shall be used as the test fluid medium.
extracting fluid from the test article for analysis.
This applies to components that have a cavity from
These test fluids may be flushing, rinsing, packing, end use
which fluid may be extracted)
operating, or suitable substitutes for end use operating fluids.
Practice B—Flowing Fluid Sampling (Method for flush- 14 – 22
(Warning—Practices for sampling surface cleanliness by the ing contaminants from the test article for analysis.
This applies to components which fluid can pass (1)
vacuum cleaner technique (used on clean room garments and
directly through, or (2) pass into and out of by cy-
large storage tanks) sampling gaseous fluids and handling
cling)
hazardous fluids such as oxidizers, acids, propellants, and so Practice C—Rinse Fluid Sampling (Method for rinsing 23 – 31
contaminants from the test article’s surfaces. The
forth, are not within the scope of the practices presented;
rinse fluid is analyzed for contamination. This ap-
however, they may be included in addendums or separate
plies to components that do not have a fluid cavity
practices at a later date. or for other reasons are not adaptable to Practices
A and B)
Substitute fluids are recommended in place of end item fluids
1.5 This standard does not purport to address all of the
for preassembly cleanliness determinations on components
safety concerns, if any, associated with its use. It is the
using hazardous end item fluids. After obtaining the sample,
responsibility of the user of this standard to establish appro-
the substitute fluid must be totally removed from the test part
with particular caution given to the possibility of trapped fluid. priate safety, health, and environmental practices and deter-
mine the applicability of regulatory limitations prior to use.
1.6 This international standard was developed in accor-
1 dance with internationally recognized principles on standard-
These practices are under the jurisdiction of ASTM Committee E21 on Space
Simulation and Applications of Space Technology and are the direct responsibility
ization established in the Decision on Principles for the
of Subcommittee E21.05 on Contamination.
Development of International Standards, Guides and Recom-
Current edition approved Oct. 1, 2016. Published September 2023. Originally
mendations issued by the World Trade Organization Technical
approved in 1965 as D2429 – 65 T. Redesignated F303 in 1970. Last previous
edition approved in 2016 as F303 – 08 (2016). DOI: 10.1520/F0303-08R23E01. Barriers to Trade (TBT) Committee.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
´1
F303 − 08 (2023)
2. Referenced Documents 3.1.10 solvent filtering dispenser, n—an apparatus to dis-
2 pense a stream of 2.0 μm or finer membrane filtered fluid.
2.1 ASTM Standards:
3.1.11 system tare, n—The measure of contamination deter-
D1836 Specification for Commercial Hexanes
mined by replacing the test component with a connecting
F311 Practice for Processing Aerospace Liquid Samples for
fitting and following the cleanliness test procedure as if
Particulate Contamination Analysis Using Membrane Fil-
checking the test component.
ters
F312 Test Methods for Microscopical Sizing and Counting
4. Summary of Practices
Particles from Aerospace Fluids on Membrane Filters
F313 Test Method for Insoluble Contamination of Hydraulic
4.1 Cleanliness is determined by sampling and analyzing
Fluids by Gravimetric Analysis (Withdrawn 1988)
fluid that has been in contact with the surface being analyzed.
Specific methods are recommended; however, other methods
2.2 Military Standards:
have been recognized due to the wide variety of components
MIL-T-27602 Trichlorine Oxygen Propellant Compatibles
MIL-H-6083 Hydraulic Fluid Petroleum Base for Pressure and different test equipment used by industry. Recommended
and alternative methods are given in Fig. 1, Fig. 2, and Fig. 3.
MIL-H-5606 Hydraulic Fluid Petroleum Base for Aircarrier
Missiles and Ordinance
PRACTICE A—STATIC FLUID SAMPLING
3. Terminology
5. Scope
3.1 Definitions:
5.1 This practice covers procedures for determining the
3.1.1 analytical membrane, n—a membrane filter used to
particulate contamination level of fluids from components that
collect the contaminant particles for analysis.
have a cavity from which fluid may be extracted.
3.1.2 azeotropic mixture, n—a solution of two or more
liquids, the composition of which does not change upon
6. Summary of Practice
distillation. Also known as azeotrope.
6.1 Fluid is extracted from the component and analyzed to
3.1.3 blank analysis, n—sometimes referred to as “fluid
determine the particulate contamination level. Recommended
tare,” “control level,” “reference contamination level,” or
and alternative methods are given in Fig. 1.
“background level.” The blank analysis is the particulate
contamination level of the test fluid when the test part is
omitted.
3.1.4 cleanup membrane, n—a membrane used to filter the
contaminant particles from the fluid medium.
3.1.5 component, n—an individual piece or a complete
assembly of individual pieces.
3.1.6 field filter holder, n—a throw-away or reusable car-
tridge containing an analytical membrane filter.
3.1.7 initial cleanliness, n—the measure of contamination
removed from the test component at the time of test, excluding
that defined by operating cleanliness.
3.1.8 membrane tare, n—sometimes referred to as “blank
count” or “control filter.” When applied to microscope
methods, the membrane tare is the quantity of particles
determined to be on the filter before the test fluid is filtered.
When applied to gravimetric methods, the membrane tare is an
amount of weight increase imparted to the control filter when
uncontaminated test fluid is passed through.
3.1.9 operating cleanliness, n—the measure of contami-
nants generated by moving parts in the component during a
specified period of dynamic operation.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website.
The last approved version of this historical standard is referenced on
www.astm.org.
Available from Standardization Documents Order Desk, Bldg. 4 Section D, 700 FIG. 1 Recommended and Alternative Methods for Static Fluid
Robbins Ave., Philadelphia, PA 19111-5098, Attn: NPODS. Sampling (Practice A)
´1
F303 − 08 (2023)
FIG. 2 Recommended and Alternative Methods for Flow Through
FIG. 3 Recommended and Alternative Methods for Rinse Fluid
Sampling (Practice B)
Sampling (Practice C)
6.2 It is recommended that all operations of this practice be
conducted in a dust controlled area. Cleanliness level of the
dust controlled area shall be consistent with the component
contamination limits.
7. Significance and Use
7.1 Although a cleaning action is imparted to the test
component, it is not the intent of this practice to serve as a
cleaning procedure. Components are normally cleaner after
each consecutive test; thus repeated tests may be used to
establish process limits for a given component (Fig. 4). A
specific set of test parameters must be supplied by the agency
FIG. 4 Contamination per Test Run Versus Consecutive Test Run
specifying cleanliness limits. Fig. 1, Fig. 2, and Fig. 3 may be
Number
used as a guide to establish the desired parameters of test fluid,
vibration, extraction, and analysis.
7.2 The curve in Fig. 4 shows the typical behavior of a
8. Apparatus
component when tested for cleanliness several consecutive
8.1 Apparatus, as described in Practice F313.
times. Stabilization generally occurs before the fifth successive
run. The stabilized region starts where a horizontal line through
8.2 Apparatus, as described in Test Methods F312 or as
the maximum stabilized value intersects the curve. described in Practice F311.
7.3 The allowable cleanliness limit of a test component
8.3 Automatic Particle Counter, as required.
should be based on the cleanliness requirements of the system
8.4 Vibration Equipment, as specified.
in which it will be used, and the assigned value should be
8.5 Apparatus Setup for Removing Component Fluid
greater than the maximum stabilized value. When defining the
Sample, as shown in Fig. 4.
allowable cleanliness limits, an important consideration is that
the accuracy of the results decreases as the allowable limit
NOTE 4—Any suitable syringe and solvent dispensing devices may be
value approaches the stabilized value. used.
´1
F303 − 08 (2023)
8.6 Apparatus Setup for Providing Filtered Fluids, as shown 9.4.8 Deionized water.
in Fig. 5 (Note 4).
5 NOTE 5—Methyl-chloroform, used in these practices, is toxic, and is
9. Reagents
being phased out for many applications. Methyl-chloroform has been
replaced in this edition of these practices. The replacement solvents were
9.1 Purity of Reagents—Reagent grade chemicals shall be
selected based on tests and analyses performed by The Aerospace
used in all tests. Unless otherwise indicated, it is intended that
Corporation and described in SMC-TR-95–28.
all reagents shall conform to the specifications of the Commit-
NOTE 6—Trichloroethylene has been labeled a potential human carcino-
tee on Analytical Reagents of the American Chemical Society,
gen by the Environmental Protection Agency. Use should be restricted to
where such specifications are available. Other grades may be
limit human exposure.
used, provided it is first ascertained that the reagent is of
10. Preparation of Apparatus
sufficiently high purity to permit its use without lessening the
accuracy of the determination.
10.1 Installation Requirements for Fig. 6—The following
requirements must be accomplished prior to and during assem-
9.2 Reagents must be compatible with the materials, fluid,
bly of the apparatus shown in Fig. 6. (Warning—All connec-
and seals used in the component and apparatus.
tions must be finger tight only.)
9.3 All reagents shall be prefiltered through a 2-μm or finer
10.1.1 Install the double valve and fluid outlet plastic tube.
absolute membrane filter prior to use unless this requirement is
10.1.2 Remove caps or plugs, or both, from the field filter
impractical due to the fluid used or sizes monitored in which
holder and place them in a covered, precleaned, petri dish.
case the user must filter as necessary.
10.1.3 Install the field filter holder onto the double valve,
9.4 Low surface tension reagents commonly used are as
taking care to place the inlet side of the field filter holder
follows:
towards the fluid being withdrawn.
9.4.1 Petroleum Ether,
10.1.4 Install fluid inlet needle onto the monitor.
9.4.2 Hexane, in accordance with Specification D1836.
(Warning—The fluid inlet needle must be precleaned prior to
9.4.3 Isopropyl Alcohol,
each usage.)
9.4.4 Fluorocarbons,
10.2 General Requirements for Fig. 6:
9.4.5 Mineral Spirits,
10.2.1 A control blank must be accomplished on the appa-
9.4.6 Trichloroethylene, in accordance with MIL-T-27602,
ratus setup before fluid is withdrawn for component fluid
and
sampling.
9.4.7 Azeotropic mixture of ethyl acetate (47 % vol) and
10.2.2 It is recommended that the field filter holders be used
cyclohexane (53 % vol).
one time only for component fluid sampling. However, clean-
ing in sufficient numbers might warrant their reuse, provided it
is first determined that the monitors are sufficiently cleaned to
A Material Safety Data Sheet (MSDS) can be obtained from the vendor. The
permit their reuse without lessening the accuracy of the
following website can also provide MSDS’s for all materials:
determination.
www.msdssearch.com/DBlinksN.htm. Note that the specific fluorocarbon must be
identified.
10.2.3 Always actuate the syringe plunger slowly when
6 6
filling or ejecting fluid.
ACS Reagent Chemicals, Specifications and Procedures for
Reagents and Standard-Grade Reference Materials, American
Chemical Society, Washington, DC. For suggestions on the
testing of reagents not listed by the American Chemical
Aerospace Corporation
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.