ASTM F2062-00(2018)
(Specification)Standard Specification for Square Drive Interconnections on Surgical Instruments
Standard Specification for Square Drive Interconnections on Surgical Instruments
ABSTRACT
This specification covers the dimensions and tolerances for both driving and driven elements in square drive interconnections on stainless steel surgical instruments used for drilling, tapping, driving, or placing of medical devices during surgery. The specification is intended to lessen the chance of accidental disengagement of surgical instruments.
SCOPE
1.1 This specification applies to interconnections of surgical instruments used for drilling, tapping, driving, or placing of medical devices during surgery.
1.2 This specification includes dimensions and tolerances for both driving and driven elements.
1.3 The specifications given in ASME B107.4M-1995 are designed for industrial applications and are considered too loose for surgical applications. Springs used for industrial applications are generally made from carbon steel and are capable of higher loads than their stainless steel counterparts. The specifications given in this standard have been written to lessen the chance of accidental disengagement of surgical instruments. This accidental disengagement could injure the patient or end user, or damage or contaminate the instrument.
1.4 The values stated in either SI units or inch-pound units are to be regarded separately as standard. The values stated in each system may not be exact equivalents; therefore, each system shall be used independently of the other. Combining values from the two systems may result in non-conformance with the standard.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F2062 −00 (Reapproved 2018)
Standard Specification for
Square Drive Interconnections on Surgical Instruments
This standard is issued under the fixed designation F2062; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2.2 Other Standard:
ASME B107.4M-1995 (Revision of ANSI B107.4-1982),
1.1 This specification applies to interconnections of surgical
Driving and Spindle Ends for Portable Hand, Impact,Air,
instruments used for drilling, tapping, driving, or placing of
and Electric Tools Percussion Tools Excluded
medical devices during surgery.
1.2 This specification includes dimensions and tolerances
3. Terminology
for both driving and driven elements.
3.1 Definitions of Terms Specific to This Standard:
1.3 The specifications given in ASME B107.4M-1995 are
3.1.1 drilling—the act of forming a hole.
designed for industrial applications and are considered too
3.1.2 driving—the act of turning, pushing, or pulling a
loose for surgical applications. Springs used for industrial
surgical instrument to place a medical device during surgery.
applications are generally made from carbon steel and are
3.1.3 square drive—a male or female interconnection with
capable of higher loads than their stainless steel counterparts.
four driving surfaces that are of equal width and perpendicular
The specifications given in this standard have been written to
to each other.
lessen the chance of accidental disengagement of surgical
3.1.4 tapping—the act of forming threads.
instruments. This accidental disengagement could injure the
patient or end user, or damage or contaminate the instrument.
4. Material
1.4 The values stated in either SI units or inch-pound units
4.1 This specification is intended to apply only to stainless
are to be regarded separately as standard. The values stated in
steel instruments that conform to Specification F899. If other
each system may not be exact equivalents; therefore, each
types of materials are used to interconnect with stainless steel
system shall be used independently of the other. Combining
instruments, then they should adhere to this specification.
values from the two systems may result in non-conformance
with the standard.
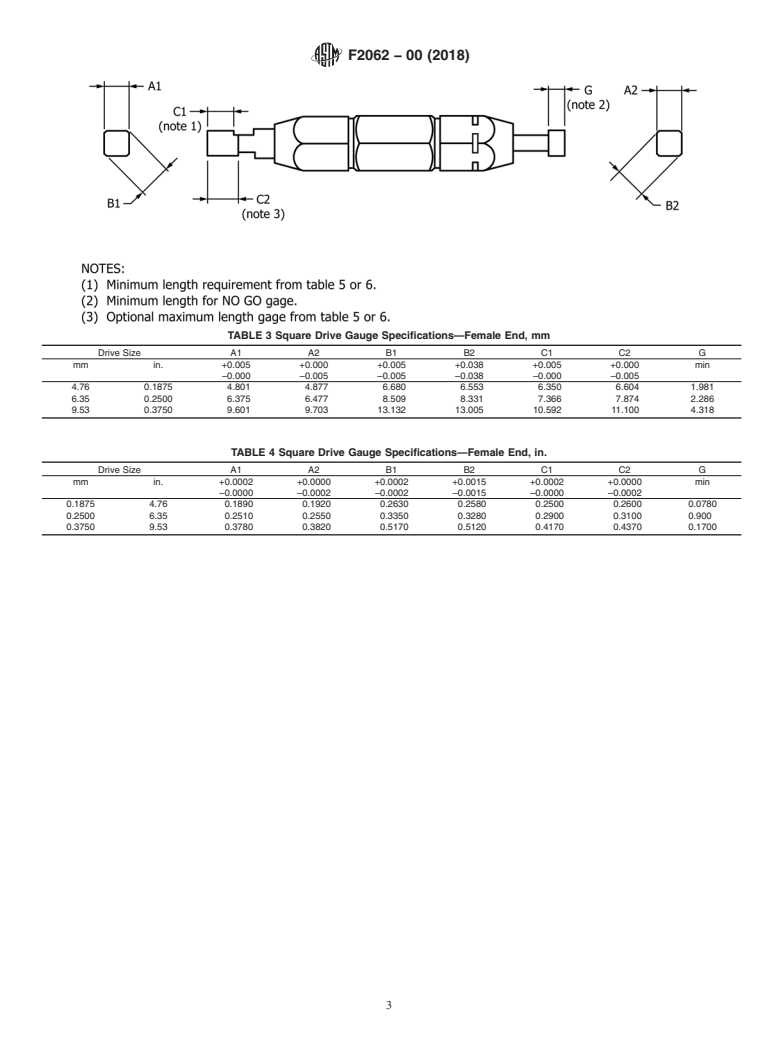
5. Gauge Use and Design
1.5 This international standard was developed in accor-
5.1 Tables1-8aredescriptiveandnotrestrictive,andarenot
dance with internationally recognized principles on standard-
intended to preclude the manufacture of product or gauges
ization established in the Decision on Principles for the
which are otherwise in accordance with this specification.
Development of International Standards, Guides and Recom-
5.2 Manufacturers may use gauges with tighter dimensions
mendations issued by the World Trade Organization Technical
or tolerances than shown herein to ensure device acceptance.
Barriers to Trade (TBT) Committee.
5.3 The extreme size for all limit (GO and NO-GO) gauges
2. Referenced Documents
shall not exceed the extreme limits of interconnections speci-
fied within this specification. All variations (manufacturing
2.1 ASTM Standards:
tolerance,calibrationerror,wearallowance,andsoforth)inthe
F899 Specification for Wrought Stainless Steels for Surgical
gauges, whatever their cause or purpose, shall bring these
Instruments
gauges within the extreme limits of the gauge size specified in
this specification. Thus, a gauge representing a minimum limit
may be larger, but never smaller, than the minimum size
This specification is under the jurisdiction of ASTM Committee F04 on
specified for the interconnection in this specification; likewise,
Medical and Surgical Materials and Devices and is the direct responsibility of
Subcommittee F04.33 on Medical/Surgical Instruments.
a gauge representing a maximum limit may be smaller, but
Current edition approved Oct. 1, 2018. Published November 2018. Originally
never larger, than the maximum size specified for the intercon-
approved in 2000. Last previous edition approved in 2011 as F2062 – 00(2011).
nection in this specification.
DOI: 10.1520/F2062–00R18.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on Available from American National Standards Institute (ANSI), 25 W. 43rd St.,
the ASTM website. 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
F2062 − 00 (2018)
TABLE 1 Square Drive Specifications—Female End, mm
Drive Size A (square
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.