ASTM E659-78(1994)e1
(Test Method)Standard Test Method for Autoignition Temperature of Liquid Chemicals
Standard Test Method for Autoignition Temperature of Liquid Chemicals
SCOPE
1.1 This test method covers the determination of hot- and cool-flame autoignition temperatures of a liquid chemical in air at atmospheric pressure in a uniformly heated vessel.
Note 1--Within certain limitations, this test method can also be used to determine the autoignition temperature of solid chemicals which readily melt and vaporize at temperatures below the test temperature.
1.2 This standard should be used to measure and describe the properties of materials, products, or assemblies in response to heat and flame under controlled laboratory conditions and should not be used to describe or appraise the fire hazard or fire risk of materials, products, or assemblies under actual fire conditions. However, results of this test may be used as elements of a fire risk assessment which takes into account all of the factors which are pertinent to an assessment of the fire hazard of a particular end use .
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
e1
Designation: E 659 – 78 (Reapproved 1994)
AMERICAN SOCIETY FOR TESTING AND MATERIALS
100 Barr Harbor Dr., West Conshohocken, PA 19428
Reprinted from the Annual Book of ASTM Standards. Copyright ASTM
Standard Test Method for
Autoignition Temperature of Liquid Chemicals
This standard is issued under the fixed designation E 659; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
e NOTE—Editorial changes were made throughout in December 1994.
INTRODUCTION
This test method is one of several methods developed by ASTM Committee E-27 for determining
the hazards of chemicals. It is designed to be used in conjunction with other tests to characterize the
hazard potential of the chemical under test.
1. Scope oxidation reaction in the absence of an external ignition source
such as a spark or flame.
1.1 This test method covers the determination of hot- and
3.3 autoignition temperature, n—the minimum temperature
cool-flame autoignition temperatures of a liquid chemical in air
at which autoignition occurs under the specified conditions of
at atmospheric pressure in a uniformly heated vessel.
test.
NOTE 1—Within certain limitations, this test method can also be used to
3.3.1 Autoignition temperature is also referred to as spon-
determine the autoignition temperature of solid chemicals which readily
taneous ignition temperature, self-ignition temperature, autog-
melt and vaporize at temperatures below the test temperature.
enous ignition temperature, and by the acronyms AIT and SIT.
1.2 This standard should be used to measure and describe
As determined by this method, AIT is the lowest temperature at
the properties of materials, products, or assemblies in response
which the substance will produce hot-flame ignition in air at
to heat and flame under controlled laboratory conditions and
atmospheric pressure without the aid of an external energy
should not be used to describe or appraise the fire hazard or
source such as spark or flame. It is the lowest temperature to
fire risk of materials, products, or assemblies under actual fire
which a combustible mixture must be raised, so that the rate of
conditions. However, results of this test may be used as
heat evolved by the exothermic oxidation reaction will over-
elements of a fire risk assessment which takes into account all
balance the rate at which heat is lost to the surroundings and
of the factors which are pertinent to an assessment of the fire
cause ignition.
hazard of a particular end use.
3.4 cool-flame, n—a faint, pale blue luminescence or flame
occurring below the autoignition temperature (AIT).
2. Referenced Documents
NOTE 2—Cool-flames occur in rich vapor-air mixtures of most hydro-
2.1 ASTM Standards:
carbons and oxygenated hydrocarbons. They are the first part of the
D 2883 Test Method for Reaction Threshold Temperature
multistage ignition process.
of Liquid and Solid Materials
3.5 ignition delay time, n—the time lapse between applica-
3. Terminology
tion of heat to a material and its ignition. It is the time in
seconds between insertion of the sample into the flask and
3.1 Definitions:
ignition. It is maximum at the minimum autoignition tempera-
3.1.1 ignition, n—the initiation of combustion.
ture and also referred to as ignition lag.
3.1.2 Ignition, which is subjective, is defined for this
method as the appearance of a flame accompanied by a sharp
4. Summary of Test Method
rise in the temperature of the gas mixture. The determination is
4.1 A small, metered sample of the product to be tested is
made in total darkness because some flames, such as cool-
inserted into a uniformly heated 500-ml glass flask containing
flames, are observed with difficulty.
air at a predetermined temperature. The contents of the flask
3.2 autoignition, n—the ignition of a material commonly in
are observed in a dark room for 10 min following insertion of
air as the result of heat liberation due to an exothermic
the sample, or until autoignition occurs. Autoignition is evi-
denced by the sudden appearance of a flame inside the flask
This test method is under the jurisdiction of ASTM Committee E-27 on Hazard
and by a sharp rise in the temperature of the gas mixture. The
Potential of Chemicals,and is the direct responsibility of Subcommittee E27.04 on
Flammability and Ignitability of Liquid Chemicals. lowest internal flask temperature (T) at which hot-flame
Current edition approved Aug. 25, 1978. Published November 1978.
ignition occurs for a series of prescribed sample volumes is
Annual Book of ASTM Standards, Vol 05.02.
E 659
taken to be the hot-flame autoignition temperature (AIT) of the uniform temperature within the flask shall be used. A furnace
chemical in air at atmospheric pressure. Ignition delay times with a cylindrically shaped interior, 5 in. (12.7 cm) in inside
(ignition time lags) are measured in order to determine the diameter, and 7 in. (17.8 cm) deep is minimal for this purpose.
ignition delay-ignition temperature relationship. It should be capable of attaining a temperature of 600°C or
4.2 The temperatures at which cool-flame ignitions are higher.
observed or evidenced by small sharp rises of the gas mixture
6.2 Temperature Controller—A temperature control system,
temperature are also recorded along with the corresponding
capable of controlling the temperature in the furnace to
ignition delay times. The lowest flask temperature at which
within6 1°C at temperatures up to 350°C, and to within 62°C
cool-flame ignition occurs is taken to be the cool-flame
above 350°C, is required. Temperatures are monitored at the
autoignition temperature (CFT). Similarly, observations are
bottom, side, and neck of the flask by means of three external
made of any nonluminous preflame reactions, as evidenced by
thermocouples. Heating adjustments are made when necessary
a relatively gradual temperature rise which then falls off to the
in order to maintain uniform temperature within the flask. If a
base temperature. The lowest flask temperature at which these
controller is not available, temperature control may be
reactions are observed is the reaction threshold temperature
achieved by the use of suitable autotransformers or rheostats,
(RTT).
thermocouples, and a suitable potentiometer.
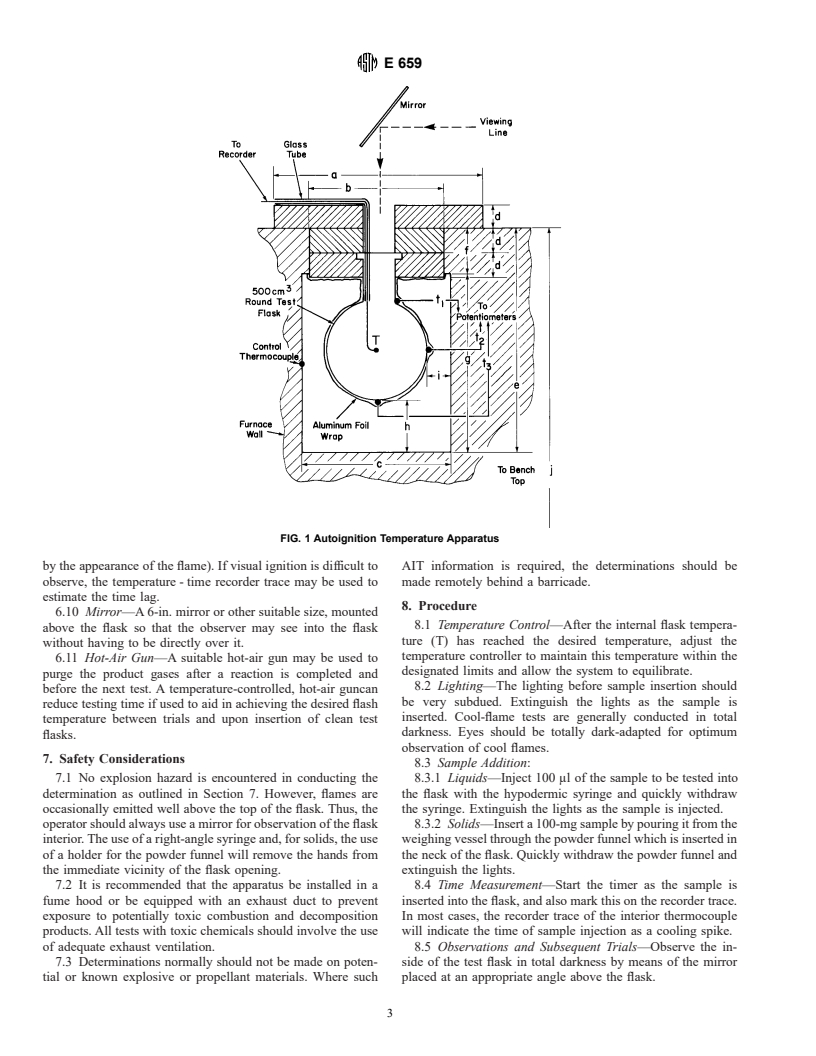
6.3 Test Flask—The test flask shall be a commercial 500-ml
NOTE 3—The hot-flame autoignition, cool-flame autoignition, and re-
borosilicate round-bottom, short-necked boiling flask.
action threshold temperatures obtained by this test method approximate
those temperatures obtained by Test Method D 2883 for hot-flame 6.3.1 The flask is closely wrapped in reflective metal foil,
reaction, cool-flame reaction, and reaction threshold, respectively.
such as aluminum, to promote temperature uniformity, and is
suspended in the furnace so as to be completely enclosed with
5. Significance and Use
the top of the neck being inset below the top of the insulated
5.1 Autoignition, by its very nature, is dependent on the
cover (see Fig. 1).
chemical and physical properties of the material and the
6.3.2 The flask is suspended in the furnace or sand bath by
method and apparatus employed for its determination. The
means of a thick insulating holder, the bottom of which is also
autoignition temperature by a given method does not necessar-
covered with reflective metal foil.
ily represent the minimum temperature at which a given
6.4 Hypodermic Syringe—A 500 or 1000-μl hypodermic
material will self-ignite in air. The volume of the vessel used is
syringe equipped with a 6-in., No. 26 or finer stainless steel
particularly important since lower autoignition temperatures
needle, and calibrated in units of 10 μl should be used to inject
will be achieved in larger vessels. (See Appendix X2.) Vessel
liquid samples into the heated flask. It is suggested that a
material can also be an important factor.
needle with a right-angle bend be used so that the operator’s
5.2 The temperatures determined by this test method are
fingers can be kept away from the flask opening.
those at which air oxidation leads to ignition. These tempera-
6.5 Balance—A laboratory balance capable of weighing to
tures can be expected to vary with the test pressure and oxygen
the nearest 10 mg shall be used for preparing samples that are
concentration.
solid at room temperature. Sample weights will range from 10
5.3 This test method is not designed for evaluating materials
to 1000 mg.
which are capable of exothermic decomposition. For such
6.6 Powder Funnel—A 60-mm filling funnel is used to aid
materials, ignition is dependent upon the thermal and kinetic
the insertion of solid samples into the flask. It is suggested that
properties of the decomposition, the mass of the sample, and
a holder such as a small buret clamp be used so that the
the heat transfer characteristics of the system.
operator’s fingers can be kept away from the flask opening.
5.4 This test method can be employed for solid chemicals
6.7 Thermocouple—A fine Chromel-Alumel thermocouple
which melt and vaporize or which readily sublime at the test
(36 B and S gage) is used for measuring the gas temperature
temperature. No condensed phase, liquid or solid, should be
(T) inside the flask. Position the tip of the thermocouple at the
present when ignition occurs.
center of the flask. Thermocouples should be calibrated against
5.5 This test method is not designed to measure the autoi-
standard temperatures or a standard thermocouple, and should
gnition temperature of materials which are solids or liquids at
be rechecked frequently. Iron-constantan thermocouples are to
the test temperature (for example, wood, paper, cotton, plastics,
be avoided because they may promote catalytic oxidation on
and high-boiling point chemicals). Such materials will ther-
the iron-oxide surface. External flask temperatures are mea-
mally degrade in the flask and the accumulated degradation
sured with a No. 20 B and S gage or finer thermocouple
products may ignite.
mounted at the top (t ), middle (t ), and bottom (t ) of the flask.
1 2 3
5.6 This test method was developed primarily for liquid
6.8 Recording Potentiometer—A fast response (1 s or less
chemicals but has been employed to test readily vaporized
for full scale pen travel) variable range and variable chart speed
solids. Responsibility for extension of this method to solids of
recording potentiometer shall be used for recording the signal
unknown thermal stability, boiling point, or degradation char-
from the internal gas thermocouple (T). An x - y recorder has
acteristics rests with the operator.
been found suitable for this purpose.
6. Apparatus
6.9 Timer—A stop watch or electric timer (preferably foot-
6.1 Furnace—An electrically heated crucible furnace or switch operated) calibrated in 0.1 or 0.2-s units shall be used to
fluidized sand bath of appropriate internal geometry and determine the time lag before ignition (time interval between
dimensions to contain the test flask and which will maintain a the instant of sample insertion and that of ignition as evidenced
E 659
FIG. 1 Autoignition Temperature Apparatus
by the appearance of the flame). If visual ignition is difficult to AIT information is required, the determinations should be
observe, the temperature - time recorder trace may be used to made remotely behind a barricade.
estimate the time lag.
8. Procedure
6.10 Mirror—A 6-in. mirror or other suitable size, mounted
8.1 Temperature Control—After the internal flask tempera-
above the flask so that the observer may see into the flask
ture (T) has reached the desired temperature, adjust the
without having to be directly over it.
temperature controller to maintain this temperature within the
6.11 Hot-Air Gun—A suitable hot-air gun may be used to
designated limits and allow the system to equilibrate.
purge the product gases after a reaction is completed and
8.2 Lighting—The lighting before sample insertion should
before the next test. A temperature-controlled, hot-air guncan
be very subdued. Extinguish the lights as the sample is
reduce testing time if used to aid in achieving the desired flash
inserted. Cool-flame tests are generally conducted in total
temperature between trials and upon insertion of clean test
darkness. Eyes should be totally dark-adapted for optimum
flasks.
observation of cool flames.
7. Safety Considerations
8.3 Sample Addition:
7.1 No explosion hazard is encountered in conducting the 8.3.1 Liquids—Inject 100 μl of the sample to be tested into
determination as outlined in Section 7. However, flames are the flask with the hypodermic syringe and quickly withdraw
occasionally emitted well above the top of the flask. Thus, the the syringe. Extinguish the lights as the sample is injected.
operator should always use a mirror for observation of the flask 8.3.2 Solids—Insert a 100-mg sample by pouring it from the
interior. The use of a right-angle syringe and, for solids, the use weighing vessel through the powder funnel which is inserted in
of a holder for the powder funnel will remove the hands from the neck of the flask. Quickly withdraw the powder funnel and
the immediate vicinity of the flask opening. extinguish the lights.
7.2 It is recommended that the apparatus be installed in a 8.4 Time Measurement—Start the timer as the sample is
fume hood or be equipped with an exhaust duct to prevent inserted into the flask, and also mark this on the recorder trace.
exposure to potentially toxic combustion and decomposition In most cases, the recorder trace of the interior thermocouple
products. All tests with toxic chemicals should involve the use will indicate the time of sample injection as a cooling spike.
of adequate exhaust ventilation. 8.5 Observations and Subsequent Trials—Observe the in-
7.3 Determinations normally should not be made on poten- side of the test flask in total darkness by means of the mirror
tial or known explosive or propellant materials. Where such placed at an appropriate angle above the flask.
E 659
8.5.1 If ignition is not observed in 10 min, consider the a few hundred degrees or more, whereas, the cool flames are
concentration of the sample tested to be nonflammable at the accompanied by rises of less than 100°C. Cool flames gener-
gas temperature in the flask (Note 4). Completely purge the ally occur at lower flask temperatures than hot flames but may
flask with the hot-air gun. Reset the timer and recorder. Repeat form over an intermediate temperature range, so that the lowest
the test at a higher temperature (about 30°C). Allow
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.