ASTM F703-96(2002)e1
(Specification)Standard Specification for Implantable Breast Prostheses
Standard Specification for Implantable Breast Prostheses
SCOPE
1.1 This specification covers the requirements for silicone gel-filled, saline inflatable silicone gel-filled, and saline inflatable, smooth-shell implantable breast prostheses intended for use in surgical reconstruction, augmentation, or replacement of the breast.
1.2 Limitations This specification does not cover custom fabricated implantable breast prostheses.
1.3 The values stated in SI units are to be regarded as the standard. The inch-pound units given in parentheses are for information only.
This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
e1
Designation: F 703 – 96 (Reapproved 2002)
Standard Specification for
Implantable Breast Prostheses
This standard is issued under the fixed designation F 703; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
e NOTE—A reference to Appendix 2 was editorially corrected in paragraph 9.1 in June 2006.
1. Scope 3.1.1 barrier coat—a silicone elastomer layer as a part of
the shell of a silicone gel implantable breast prostheses that
1.1 This specification covers the requirements for silicone
retards silicone bleed.
gel-filled, saline inflatable silicone gel-filled, and saline inflat-
3.1.2 fixation site—an area of the shell of an implantable
able, smooth-shell implantable breast prostheses intended for
breast prosthesis containing material that allows tissue in-
use in surgical reconstruction, augmentation, or replacement of
growth.
the breast.
3.1.3 fused or adhered joints (seams)—sites in the shell or
1.2 Limitations—This specification does not cover custom
other parts of an implantable breast prosthesis where materials
fabricated implantable breast prostheses.
havebeenjoined(fusedorbonded)together,withorwithoutan
1.3 The values stated in SI units are to be regarded as the
adhesive, as part of the manufacturing process.
standard. The inch-pound units given in parentheses are for
3.1.4 gel bleed—diffusion of liquid silicone components of
information only.
silicone gel through the shell of an implantable breast prosthe-
1.4 This standard does not purport to address all of the
sis.
safety concerns, if any, associated with its use. It is the
3.1.5 gel filled breast prosthesis—implantable breast pros-
responsibility of the user of this standard to establish appro-
thesis designed and provided with a pre-filled, fixed volume of
priate safety and health practices and determine the applica-
silicone gel.
bility of regulatory limitations prior to use.
3.1.5.1 Type I—afixedvolumegelfilledbreastprosthesis—
2. Referenced Documents implantable breast prosthesis comprised of a single lumen
containing a fixed amount of silicone gel. The lumen of Type
2.1 ASTM Standards:
I breast prostheses is not accessible for volume adjustments of
D 412 Test Methods for Vulcanized Rubber and Thermo-
any kind.
plastic Elastomers—Tension
3.1.5.2 Type II—double lumen inflatable gel filled breast
D 1349 Practice for Rubber—Standard Temperatures for
prosthesis—an implantable breast prosthesis comprised of two
Testing
complete lumens, one inside the other. The inner lumen
F 604 Specification for Silicone Elastomers Used in Medi-
containsafixedamountofsiliconegelandisnotaccessiblefor
cal Applications
volume adjustments of any kind. The outer lumen is provided
F 748 Practice for Selecting Generic Biological Test Meth-
with a valve to facilitate filling the void between the inner and
ods for Materials and Devices
outer lumens with saline to adjust the total volume of the
F 1251 Terminology Relating to Polymeric Biomaterials in
prosthesis, only at the time of use.
Medical and Surgical Devices
3.1.5.3 Type III—reverse double lumen inflatable gel filled
3. Terminology
breast prosthesis—an implantable breast prosthesis comprised
of two complete lumens, one inside the other. The volume
3.1 Definitions:
between the inner and outer lumens contains a fixed amount of
siliconegelandisnotaccessibleforvolumeadjustmentsofany
This specification is under the jurisdiction of ASTM Committee F04 on
kind. The inner lumen is contained within the silicone gel
Medical and Surgical Materials and Devices and is the direct responsibility of
contained in the outer lumen and has a valve system to
Subcommittee F04.32 on Plastic and Reconstructive Surgery.
facilitate filling the inner lumen with saline to increase the
Current edition approved June 10, 1996. Published May 1997. Originally
published as F 703 – 81. Last previous edition F 703 – 81 (1986). volume of the prosthesis at the time of use. The valve system
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
is also designed to facilitate post-operative volume adjustment
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
by following the instructions provided in product literature.
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website.
Withdrawn.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
e1
F 703 – 96 (2002)
3.1.6 inflatable breast prosthesis—implantable breast pros- 4.1.2 Barrier Coatings—The following are suitable compo-
theses not containing silicone gel—implantable breast prosthe- sitions of for use in barrier coat elastomers:
ses designed and provided empty and to be filled, all or in part,
Polymer composition FVMQ or VMP M Q
2 2
Fillers B or C
with saline at the time of use to adjust the volume of the
Catalyst/cure J or K
prosthesis.
NOTE 1—The compositions listed in this section are not intended to
3.1.6.1 Type 1—fixed volume inflatable breast prosthesis,an
limitthecompositionthatmaybeusedprovidingallotherrequirementsof
implantable breast prosthesis comprised of a single lumen,
this specification are satisfied.
empty when supplied and having a valve to facilitate filling the
lumen with the entire volume of saline at the time of use.
4.1.3 Fabrication—Fabrication techniques must necessarily
3.1.6.2 Type II—adjustable inflatable breast prosthesis,an be varied depending on the type of elastomer, the portion of an
implantable breast prosthesis comprised of a single lumen, implantable breast prosthesis fabricated, its shape and its
empty when supplied and having a valve to facilitate filling the location and function on the prosthesis.
lumen with a system is designed to facilitate further post-
4.1.4 Vulcanization and Postcure—Timeandtemperatureof
operative adjustment with saline as instructed in product
vulcanizationandpostcuremustbeadjustedwithconsideration
literature.
of the elastomer type and the multi-step fabrication require-
3.1.7 low bleed—silicone gel implantable breast prostheses ments of specific prostheses. Final postcure is typically done
designed to have minimal silicone bleed when tested by the only after the shell or shells and all other portions have been
method in 9.1.1. completely assembled. Time and temperature of final postcure
3.1.8 lumen—a cavity within a shell of an implantable shall be adequate to drive the chemistry of vulcanization of all
elastomers to completion and remove by-products of the cure
breast prosthesis. A lumen may contain either a fixed, non-
adjustablevolumeofsiliconegel,oritmaybeentirelyorpartly in keeping with the chemical stoichiometry of the specific cure
systems (for example, after postcure no additional vulcaniza-
empty and intended to be inflated (filled) with saline. Inflatable
lumens are accessible by valve to facilitate the addition of tion should occur when heated additionally at recommended
cure temperature).
saline to adjust the volume of the prosthesis at the time of use.
More than one lumen may be formed within a shell by silicone
4.1.5 Physical Property Testing and Requirements—
elastomer membrane partitions. Silicone elastomer shells shall demonstrate an acceptable
response in physical property tests. Prostheses for testing
3.1.9 orientation means—any mark or palpable portion of
should be selected from standard production batches which
an implantable breast prosthesis to assist the surgeon in
have gone through all manufacturing processes, including
positioning the implant.
sterilization.Withsiliconegelprostheses,removegelandclean
3.1.10 saline—only sodium chloride injection USP is rec-
shell with appropriate polar (for example, 2-propanol) or
ommended for filling lumens of implantable breast prosthesis.
non-polar (aliphatic, aromatic, or chlorinated hydrocarbon)
3.1.11 shell—asiliconeelastomercontinuouslayerormem-
solvent, or both. If solvent cleaned, condition shell afterwards
brane container (sac) that encloses a lumen or multiple lumens
for 3 h 150°F (65.6°C) in an air circulation oven to remove
of an implantable breast prosthesis.
solvent.
3.1.12 silicone elastomer—an elastomer containing cross-
4.1.5.1 Specimen Preparation—Cut required tensile test
linked silicone polymer and fumed amorphous (non-
specimens from shells with Test Methods D 412 dies. Speci-
crystalline) silica as a reinforcing filler.
mens shall be conditioned before testing for at least3hat23
3.1.13 silicone gel—a semisolid material consisting of a
6 2°C (73.4 6 3.6°F).
crosslinked silicone polymer network in which liquid silicone
4.1.6 Test Procedure—Unlessotherwisespecified,thestan-
polymer is held (see definition of gel in Terminology F 1251).
dard temperature for testing shall be 23 6 2°C (73.4 6 3.6°F).
3.1.14 valve—user sealable or self sealing opening in an
When testing at any other temperature is required, use one of
inflatable or gel saline prosthesis, extending from the exterior
the temperatures specified in Practice D 1349. Requirements
surface of the shell into a lumen, designed to facilitate addition
are as follows:
of saline at the time of use to fill the prosthesis and increase
4.1.6.1 Percent Elongation—Percent elongation shall be
prosthesis volume.
350 % minimum when tested in accordance withTest Methods
D 412.
4. Materials and Manufacture
4.1.6.2 Breaking Strength—Ultimate breaking force in ten-
4.1 Silicone elastomer—Select and specify elastomers for
sion shall be no less than 11.12 N (2.5 lbs) when tested in
use in implantable breast prostheses in keeping with Specifi-
accordance with Test Methods D 412.
cation F 604.
4.1.6.3 Tensile Set— Determine tensile set at 300 % elon-
4.1.1 Shell—The following describes suitable silicone elas-
gation, stress the specimen for 3 min then allow 3 min for
tomer compositions for use as the primary material of con-
relaxation. The tensile set shall be <10 %, determined in
struction of the shell including the exterior (tissue contact)
accordance with Test Methods D 412.
surface:
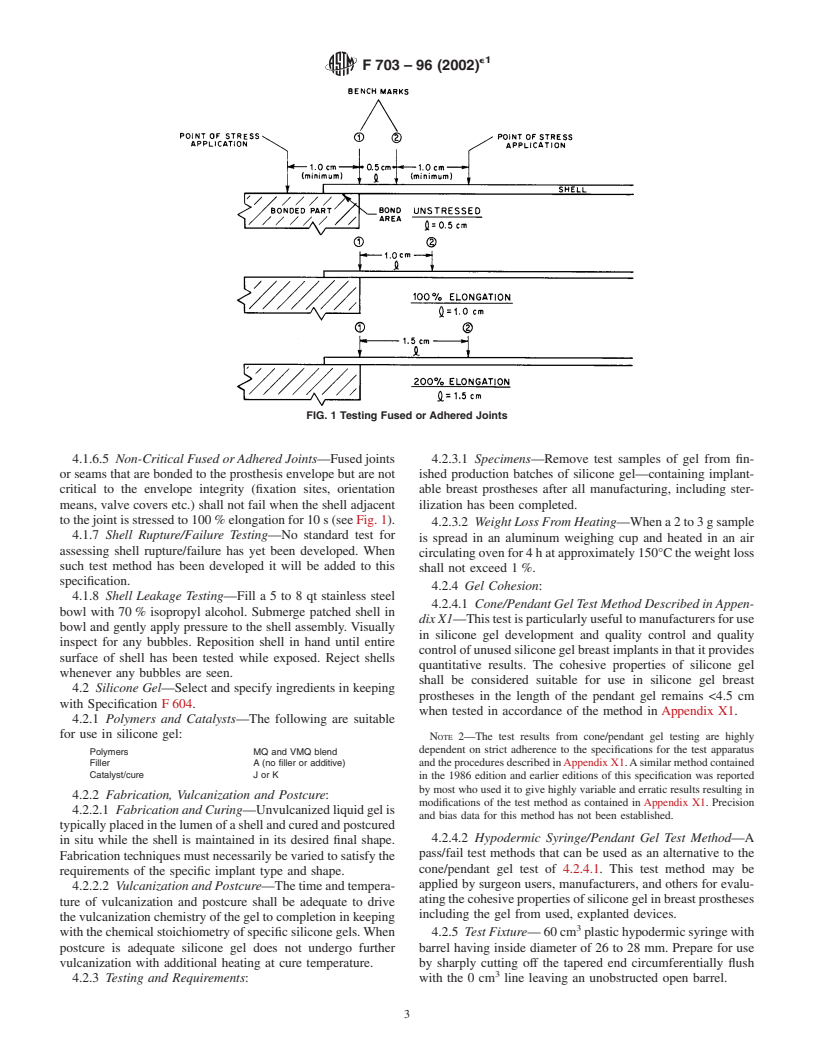
4.1.6.4 Critical Fused or Adhered Joints—Joints or seams
Polymer types MQ or VMQ
that are critical to the integrity of the prosthesis envelope shall
Fillers A, B or C
Additive J (for radiopacity)
not fail when the shell adjacent to the joint is stressed to 200 %
Catalysts B, G, J, or K
elongation for 10 s (see Fig. 1).
e1
F 703 – 96 (2002)
FIG. 1 Testing Fused or Adhered Joints
4.1.6.5 Non-Critical Fused or Adhered Joints—Fused joints 4.2.3.1 Specimens—Remove test samples of gel from fin-
or seams that are bonded to the prosthesis envelope but are not ished production batches of silicone gel—containing implant-
critical to the envelope integrity (fixation sites, orientation able breast prostheses after all manufacturing, including ster-
means, valve covers etc.) shall not fail when the shell adjacent ilization has been completed.
tothejointisstressedto100 %elongationfor10s(seeFig.1).
4.2.3.2 Weight Loss From Heating—Whena2to3gsample
4.1.7 Shell Rupture/Failure Testing—No standard test for
is spread in an aluminum weighing cup and heated in an air
assessing shell rupture/failure has yet been developed. When
circulatingovenfor4hatapproximately150°Ctheweightloss
such test method has been developed it will be added to this
shall not exceed 1 %.
specification.
4.2.4 Gel Cohesion:
4.1.8 Shell Leakage Testing—Filla5to8qt stainless steel
4.2.4.1 Cone/Pendant Gel Test Method Described inAppen-
bowl with 70 % isopropyl alcohol. Submerge patched shell in
dixX1—Thistestisparticularlyusefultomanufacturersforuse
bowl and gently apply pressure to the shell assembly. Visually
in silicone gel development and quality control and quality
inspect for any bubbles. Reposition shell in hand until entire
controlofunusedsiliconegelbreastimplantsinthatitprovides
surface of shell has been tested while exposed. Reject shells
quantitative results. The cohesive properties of silicone gel
whenever any bubbles are seen.
shall be considered suitable for use in silicone gel breast
4.2 Silicone Gel—Select and specify ingredients in keeping
prostheses in the length of the pendant gel remains <4.5 cm
with Specification F 604.
when tested in accordance of the method in Appendix X1.
4.2.1 Polymers and Catalysts—The following are suitable
for use in silicone gel:
NOTE 2—The test results from cone/pendant gel testing are highly
dependent on strict adherence to the specifications for the test apparatus
Polymers MQ and VMQ blend
Filler A (no filler or additive) andtheproceduresdescribedinAppendixX1.Asimilarmethodcontained
Catalyst/cure J or K
in the 1986 edition and earlier editions of this specification was reported
by most who used it to give highly variable and erratic results resulting in
4.2.2 Fabrication, Vulcanization and Postcure:
modifications of the test method as contained in Appendix X1. Precision
4.2.2.1 FabricationandCuring—Unvulcanizedliquidgelis
and bias data for this method has not been established.
typicallyplacedinthelumenofashellandcuredandpostcured
4.2.4.2 Hypodermic Syringe/Pendant Gel Test Method—A
in situ while the shell is maintained in its desired final shape.
pass/fail test methods that can be used as an alternative to the
Fabricationtechniquesmustnecessarilybevariedtosatisfythe
cone/pendant gel test of 4.2.4.1. This test method may be
requirements of the specific implant type and shape.
applied by surgeon users, manufacturers, and others for evalu-
4.2.2.2 Vulcanization and Postcure—Thetimeandtempera-
atingthecohesivepropertiesofsiliconegelinbreastprostheses
ture of vulcanization and postcure shall be adequate to drive
including the gel from used, explanted devices.
thevulcanizationchemistryofthegeltocompletioninkeeping
withthechemicalstoichiometryofspecificsiliconegels.When 4.2.5 TestFixture—60cm plastichypodermicsyringewith
postcure is adequate silicone gel does not undergo further barrel having inside diameter of 26 to 28 mm. Prepare for use
vulcanization with additional heating at cure temperature. by sharply cutting off the tapered end circumferentially flush
4.2.3 Testing and Requirements: with the 0 cm line leaving an unobstructed open barrel.
e1
F 703 – 96 (2002)
4.2.6 Test Sample—Cut the shell of the prosthesis to be 7. Fixation Sites
tested diametrally across the apex from edge to edge. Push the
7.1 Thepresenceoffixationsitesonanytypeofimplantable
plunger of the syringe forward until it is flush with the cut end
breast prosthesis is optional.When used, the size and locations
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.