ASTM F1441-03(2022)
(Specification)Standard Specification for Soft-Tissue Expander Devices
Standard Specification for Soft-Tissue Expander Devices
ABSTRACT
This specification covers the requirements for single use saline inflatable, smooth and textured tissue expansion devices to be used intraoperatively or implanted for typically less than 6 months and then removed. This specification applies only to soft-tissue expander devices fabricated with elastomer shells. It does not necessarily cover any custom fabricated soft tissue expander device manufactured to any other specification. The device shall be classified as: Type I; Type II; and Type III. Biocompatibility, tensile set, breaking force, tubing shell junction, injection port competence, overexpansion, tubing length adapter strength, needle stop penetration, and fused or adhered joints tests shall be performed to conform with the specified requirements.
SIGNIFICANCE AND USE
5.1 This specification contains requirements based on state-of-the-art science and technology as applicable to various considerations that have been identified as important to ensure reasonable safety and efficacy as it relates to the biocompatibility and the mechanical integrity of the device components in soft-tissue expander devices.
5.1.1 This specification is not intended to limit the science and technology that may be considered and applied to ensure performance characteristics of subject devices in intended applications. When new information becomes available or changes in state-of-the-art science and technology occur and relevance to subject devices has been established by valid science, it is intended that this specification will be revised in accordance with ASTM guidelines.
SCOPE
1.1 This specification covers the requirements for single-use saline inflatable, smooth and textured tissue expansion devices to be used intraoperatively or implanted for typically less than six months and then removed.
1.2 Limitations:
1.2.1 This specification applies only to soft-tissue expander devices fabricated with elastomer shells. It does not necessarily cover any custom fabricated soft tissue expander device manufactured to any other specification.
1.2.2 This specification applies in part to combination “expander/mammary” devices as classified in Section 4.
1.3 The values stated in SI units are to be regarded as standard; values in parentheses are for information only.
1.4 The following statement pertains only to the test methods and requirements portion, Section 9, of this specification: This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F1441 −03 (Reapproved 2022)
Standard Specification for
Soft-Tissue Expander Devices
This standard is issued under the fixed designation F1441; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope canized Rubber and Thermoplastic Elastomers
D1349 Practice for Rubber—Standard Conditions for Test-
1.1 Thisspecificationcoverstherequirementsforsingle-use
ing
saline inflatable, smooth and textured tissue expansion devices
F703 Specification for Implantable Breast Prostheses
to be used intraoperatively or implanted for typically less than
F748 PracticeforSelectingGenericBiologicalTestMethods
six months and then removed.
for Materials and Devices
1.2 Limitations:
F1251 Terminology Relating to Polymeric Biomaterials in
1.2.1 This specification applies only to soft-tissue expander
Medical and Surgical Devices (Withdrawn 2012)
devicesfabricatedwithelastomershells.Itdoesnotnecessarily
F2038 GuideforSiliconeElastomers,Gels,andFoamsUsed
cover any custom fabricated soft tissue expander device
in Medical Applications Part I—Formulations and Un-
manufactured to any other specification.
cured Materials
1.2.2 This specification applies in part to combination
F2042 GuideforSiliconeElastomers,Gels,andFoamsUsed
“expander/mammary” devices as classified in Section 4.
in Medical Applications Part II—Crosslinking and Fabri-
cation
1.3 The values stated in SI units are to be regarded as
standard; values in parentheses are for information only. F2051 Specification for Implantable Saline Filled Breast
Prosthesis
1.4 The following statement pertains only to the test meth-
2.2 Other Documents:
ods and requirements portion, Section 9, of this specification:
Federal Register Title 21, Part 820
This standard does not purport to address all of the safety
USP (United States Pharmacopoeia)
concerns, if any, associated with its use. It is the responsibility
Association for the Advance of Medical Instrumentation:
of the user of this standard to establish appropriate safety,
ANSI/AAMI/ISO 10993-1 Biological Testing of Medical
health, and environmental practices and determine the appli-
and Dental Materials and Devices—Part 1: Guidance on
cability of regulatory limitations prior to use.
Selection of Tests
1.5 This international standard was developed in accor-
dance with internationally recognized principles on standard- ANSI/AAMI/ST50 Dry Heat (Heated Air) Sterilizers
ANSI/AAMI/ISO 11135 Medical Devices—Validation and
ization established in the Decision on Principles for the
Development of International Standards, Guides and Recom- Routine Control of Ethylene Oxide Sterilization
ANSI/AAMI/ISO 11137 Sterilization of Health Care
mendations issued by the World Trade Organization Technical
Barriers to Trade (TBT) Committee. Products—Requirements for Validation and Routine and
Routine Control—Radiation Sterilization
2. Referenced Documents
ANSI/AAMI/ISO 11134 Sterilization of Health Care
Products—Requirements for Validation and Routine
2.1 ASTM Standards:
Control—Industrial Moist Heat Sterilization
D412 Test Methods forVulcanized Rubber andThermoplas-
Parenteral Drug Association 1981 Technical Report No. 3,
tic Elastomers—Tension
Validation of Dry Heat Processes Used for Sterilization
D624 Test Method for Tear Strength of Conventional Vul-
1 3
This specification is under the jurisdiction of ASTM Committee F04 on The last approved version of this historical standard is referenced on
Medical and Surgical Materials and Devices and is the direct responsibility of www.astm.org.
Subcommittee F04.32 on Plastic and Reconstructive Surgery. AvailablefromU.S.GovernmentPrintingOfficeSuperintendentofDocuments,
Current edition approved Oct. 1, 2022. Published October 2022. Originally 732 N. Capitol St., NW, Mail Stop: SDE, Washington, DC 20401, http://
approved in 1992. Last previous edition approved in 2014 as F1441 – 03 (2014). www.access.gpo.gov.
DOI: 10.1520/F1441-03R22. United States Pharmacopeia, Vol XXI, Mack Publishing Company, Easton, PA
For referenced ASTM standards, visit the ASTM website, www.astm.org, or 1989. Available from Pharmacopeia Convention, Inc., 12601 Twinbrook Parkway,
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Rockville, NC 00852.
Standards volume information, refer to the standard’s Document Summary page on Available fromAmerican National Standards Institute (ANSI), 25 W. 43rd St.,
the ASTM website. 4th Floor, New York, NY 10036, http://www.ansi.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
F1441 − 03 (2022)
and Depyrogenation 4.2 Type II: Immediate Tissue Expansion Device—A soft-
tissue expander device only intended for intraoperative use.
3. Terminology
4.3 Type III: Combination Expander/Mammary Device—A
3.1 Definitions: specific type of soft-tissue expander device intended to be
3.1.1 injection port—the port through which an injection to implanted for postoperative expansion of the breast and further
inflate or deflate the variable volume device is made. indicated for long-term implantation as a breast prosthesis.
4.3.1 Gel/Saline—Expansion indications for devices of this
3.1.1.1 remote port—a port that is remote from the shell and
type shall confirm to this specification in addition to Specifi-
attached to the shell by means of tubing.
cation F703, as applicable.
3.1.1.2 self-contained (integrated) port—a port that is inte-
4.3.2 Saline Only—Expansionindicationsfordevicesofthis
gral to the device shell.
type shall confirm to this specification in addition to Specifi-
3.1.2 injection surface—the area of the injection port rec-
cation F2051, as applicable.
ommended by the manufacturer for needle insertion to inflate
5. Significance and Use
or deflate the device.
5.1 This specification contains requirements based on state-
3.1.3 needle stop—the injection port component used to
limit hypodermic needle penetration through the port. of-the-art science and technology as applicable to various
considerations that have been identified as important to ensure
3.1.4 silicone elastomer—an elastomer containing cross-
reasonable safety and efficacy as it relates to the biocompat-
linked silicone polymer and fumed amorphous (non-
ibility and the mechanical integrity of the device components
crystalline) silica as a reinforcing filler.
in soft-tissue expander devices.
3.1.5 reinforced silicone elastomer—a composite of silicone
5.1.1 This specification is not intended to limit the science
elastomer and an embedded textile made from polyethylene
and technology that may be considered and applied to ensure
terephthalate (such as Dacron (trademark)) fibers.
performance characteristics of subject devices in intended
3.1.6 shell—a silicone elastomer continuous layer or mem-
applications. When new information becomes available or
brane container (sac) which encloses a lumen of a soft-tissue
changes in state-of-the-art science and technology occur and
expander.
relevance to subject devices has been established by valid
science, it is intended that this specification will be revised in
3.1.7 patch or base—a piece of silicone elastomer or rein-
forced silicone elastomer, which covers and seals the hole accordance with ASTM guidelines.
which results from the manufacturing process of shell fabrica-
6. Volume and Dimensions
tion.
6.1 Volumes of Devices—The designed or minimum and
3.1.8 lumen—a cavity within a shell and patch or base,
maximum recommended volume of saline fill shall be listed in
accessible by an injection port, to facilitate the addition of
instructions for use.
saline to adjust the volume of the soft tissue expander.
6.2 Dimensions—Therangesofshapes,volumes,basesizes,
3.1.9 tubing length adapter—the tissue expander compo-
and anterior projections are determined by the manufacturer.
nent used to connect more than one piece of remote port
Pertinent information shall be contained in the package insert.
tubing.
3.1.10 tubing/shell junction—thejunctionoftheremoteport
7. Fixation Sites
tubing to the shell of the tissue expander.
7.1 The presence of fixation sites on any type of soft-tissue
3.1.11 fused or adhered joints (seams)—sites in the shell or
expander device is optional. When used, the size and locations
other parts of the tissue expander device where materials have
of fixation sites shall be clearly stated in instructions for use.
been joined (fused or bonded) together, with or without
adhesive, as part of the manufacturing process. 8. Orientation Means
3.1.12 orientation means—any mark or palpable portion of
8.1 Orientation means are optional features of subject de-
a soft-tissue expander to assist the surgeon in positioning.
vices. When orientation means are claimed, the location and
recommended techniques for use shall be clearly described in
3.1.13 saline—only sodium chloride for injection (USP) is
instructions for use.
recommended for filling lumens of soft-tissue expanders.
3.2 For other terms used in this specification, see Terminol-
9. Test Methods and Requirements
ogy F1251.
9.1 Biocompatibility:
9.1.1 Practice F748—New or existing materials shall be in
4. Classification
compliance with Practice F748 or other accepted standards
4.1 Type I: Chronic Tissue Expansion Device—Asoft-tissue
such as ANSI/AAMI/ISO 10993-1. Assays recommended by
expander device intended to be inflated postoperatively.
Practice F748 include cell culture cytotoxicity assays; short-
term intramuscular implantation assay; short-term subcutane-
ous assay; carcinogenicity; long-term implant test; systemic
injection (acute toxicity) assay; sensitization assay; mutagen-
Available from the Parenteral Drug Association, 3 Bethesda Medical Center,
Suite 1500, Bethesda, MD 20814. icity; and pyrogenicity.
F1441 − 03 (2022)
9.1.2 Soft-Tissue Expander Devices—Test specimens for with the exception of sample thickness and cycle time. Maxi-
chronic implantation assays (carcinogenicity and long-term mum set shall be less than 10 %.
implanttests)shallbefabricatedfromthesamecombinationof
9.2.2.2 Breaking Force—Test ultimate breaking force in
silicone elastomer and by the same or similar procedures and
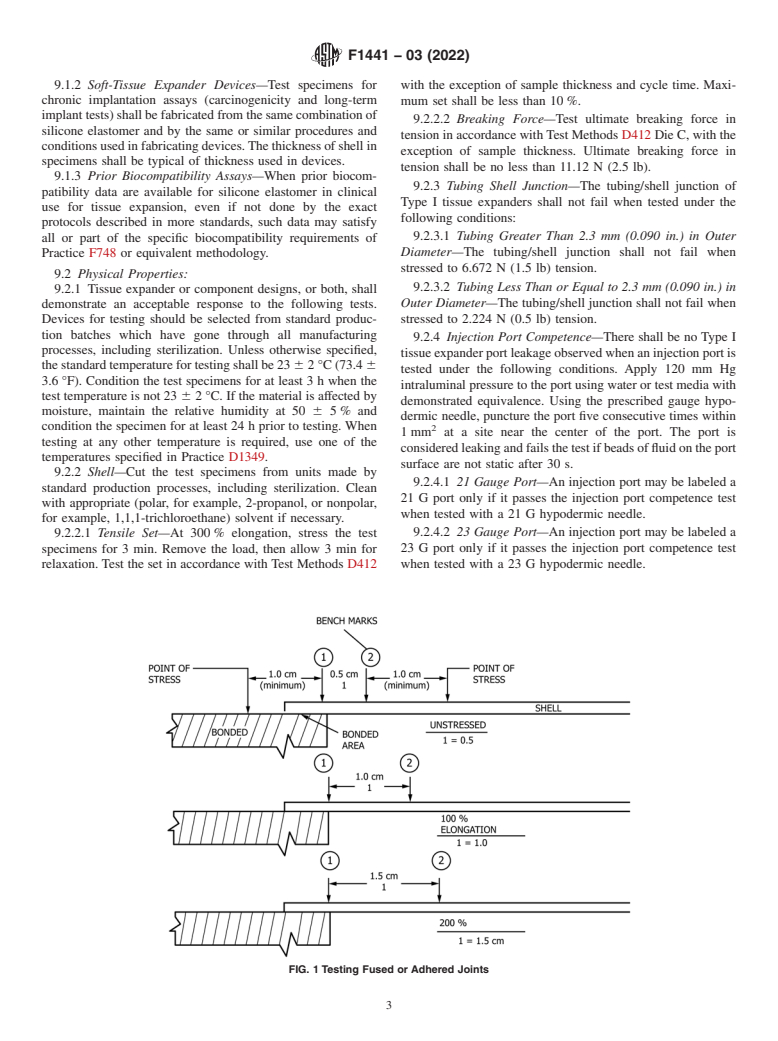
tension in accordance withTest Methods D412 Di
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.