ASTM D7649-10(2017)
(Test Method)Standard Test Method for Determination of Trace Carbon Dioxide, Argon, Nitrogen, Oxygen and Water in Hydrogen Fuel by Jet Pulse Injection and Gas Chromatography/Mass Spectrometer Analysis
Standard Test Method for Determination of Trace Carbon Dioxide, Argon, Nitrogen, Oxygen and Water in Hydrogen Fuel by Jet Pulse Injection and Gas Chromatography/Mass Spectrometer Analysis
SIGNIFICANCE AND USE
5.1 Low operating temperature fuel cells such as proton exchange membrane fuel cells (PEMFCs) require high purity hydrogen for maximum performance. The following are the reported effects (SAE TIR J2719) of the compounds determined by this test method.
5.2 Carbon Dioxide (CO2), acts largely as a diluent, however in the fuel cell environment CO2 can be transformed into CO.
5.3 Water (H2O), is an inert impurity, as it does not affect the function of a fuel cell stack; however, it provides a transport mechanism for water-soluble contaminants, such as Na+ or K+. In addition, it may form ice on valve internal surface at cold weather or react exothermally with metal hydride used as hydrogen fuel storage.
5.4 Inert Gases (N2 and Ar), do not normally react with a fuel cell components or fuel cell system and are considered diluents. Diluents can decrease fuel cell stack performance.
5.5 Oxygen (O2), in low concentrations is considered an inert impurity, as it does not adversely affect the function of a fuel cell stack; however, it is a safety concern for vehicle on board fuel storage as it can react violently with hydrogen to generate water and heat.
SCOPE
1.1 This test method describes a procedure primarily for the determination of carbon dioxide, argon, nitrogen, oxygen and water in high pressure fuel cell grade hydrogen by gas chromatograph/mass spectrometer (GC/MS) with injection of sample at the same pressure as sample without pressure reduction, which is called “Jet Pulse Injection”. The procedures described in this method were designed to measure carbon dioxide at 0.5micromole per mole (ppmv), Argon 1 ppmv, nitrogen 5 ppmv and oxygen 2 ppmv and water 4 ppmv.
1.2 The values stated in SI units are standard. The values stated in inch-pound units are for information only.
1.3 The mention of trade names in standard does not constitute endorsement or recommendation for use. Other manufacturers of equipment or equipment models can be used.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D7649 − 10 (Reapproved 2017)
Standard Test Method for
Determination of Trace Carbon Dioxide, Argon, Nitrogen,
Oxygen and Water in Hydrogen Fuel by Jet Pulse Injection
and Gas Chromatography/Mass Spectrometer Analysis
This standard is issued under the fixed designation D7649; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3. Terminology
1.1 Thistestmethoddescribesaprocedureprimarilyforthe 3.1 Definitions of Terms Specific to This Standard:
determination of carbon dioxide, argon, nitrogen, oxygen and 3.1.1 absolute pressure—pressure measured with reference
water in high pressure fuel cell grade hydrogen by gas
to absolute zero pressure, usually expressed as kPa, mm Hg,
chromatograph/mass spectrometer (GC/MS) with injection of bar or psi. All the pressures mentioned in this method are
sample at the same pressure as sample without pressure
absolute pressure.
reduction,whichiscalled“JetPulseInjection”.Theprocedures
3.1.2 constituent—A component (or compound) found
described in this method were designed to measure carbon
within a hydrogen fuel mixture.
dioxide at 0.5micromole per mole (ppmv), Argon 1 ppmv,
3.1.3 contaminant—impuritythatadverselyaffectsthecom-
nitrogen 5 ppmv and oxygen 2 ppmv and water 4 ppmv.
ponents within the fuel cell system or the hydrogen storage
1.2 The values stated in SI units are standard. The values
system by reacting with its components.An adverse effect can
stated in inch-pound units are for information only.
be reversible or irreversible.
1.3 The mention of trade names in standard does not
3.1.4 dynamic calibration—calibration of an analytical sys-
constitute endorsement or recommendation for use. Other
temusingcalibrationgasstandardgeneratedbydilutingknown
manufacturersofequipmentorequipmentmodelscanbeused.
concentration compressed gas standards with hydrogen, as
used in this method for carbon dioxide, argon, nitrogen and
1.4 This standard does not purport to address all of the
oxygen (7.3 and 7.4).
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
3.1.5 extracted ion chromatogram (EIC)—a GC/MS chro-
priate safety and health practices and determine the applica-
matogram where a selected ion is plotted to determine the
bility of regulatory limitations prior to use.
compound(s) of interest.
1.5 This international standard was developed in accor-
3.1.6 fuel cell grade hydrogen—hydrogen satisfying the
dance with internationally recognized principles on standard-
specifications in SAE TIR J2719.
ization established in the Decision on Principles for the
3.1.7 hydrogen fuel—hydrogen to be tested without compo-
Development of International Standards, Guides and Recom-
sitional change due to sample introduction, etc.
mendations issued by the World Trade Organization Technical
3.1.8 jet pulse injection—high pressure hydrogen fuel
Barriers to Trade (TBT) Committee.
sample is introduced instantaneously at the same pressure into
2. Referenced Documents GC/MS.
3.1.9 relative humidity—ratio of actual pressure of existing
2.1 Other Standards:
water vapor to maximum possible pressure of water vapor in
SAE TIR J2719Information Report on the Development of
the atmosphere at the same temperature, expressed as a
aHydrogenQualityGuidelineforFuelCellVehiclesApril
percentage.
3.1.10 response factor (RF)—-theamountinvolume(µL)of
1 an analyte divided by the EIC area of the analyte.
ThistestmethodisunderthejurisdictionofASTMCommitteeD03onGaseous
Fuels and is the direct responsibility of Subcommittee D03.14 on Hydrogen and
3.1.11 static calibration—calibration of an analytical sys-
Fuel Cells.
tem using standards in a matrix, state or manner different than
Current edition approved April 1, 2017. Published April 2017. Originally
approved in 2010. Last previous edition approved in 2010 as D7649-10. DOI: the samples to be analyzed, as used in this method for water
10.1520/D7649–10R17.
concentration in hydrogen.
AvailablefromSAEInternational(SAE),400CommonwealthDr.,Warrendale,
PA 15096-0001, http://aerospace.sae.org. 3.2 Acronyms:
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D7649 − 10 (2017)
3.2.1 FCV—fuel cell vehicle. the pressures of hydrogen standards or samples. Consequently
itisunnecessarytoregulatestandardsandhydrogensamplesto
3.2.2 PEMFC—proton exchange membrane fuel cell.
the same pressure. In addition to possible trace leaks or air
4. Summary of Test Method trappedinside,regulatorsarenotrecommendedasmoistureon
the regulator surface can be released into the sample resulting
4.1 The simultaneous analysis of carbon dioxide, argon,
in a high moisture determination.
nitrogen, oxygen and water at 0.5 – 5 ppmv (micromole per
mole) in hydrogen fuel samples from fueling stations is 4.4 A mass spectrometer provides sensitive and selective
challenging due to high hydrogen fuel sample pressure and detection towards carbon dioxide, argon, nitrogen, oxygen and
possible contaminations from ambient air. water.
4.2 In this method, a small stainless steel loop is initially
5. Significance and Use
pressurized with high pressure hydrogen standard or sample
5.1 Low operating temperature fuel cells such as proton
without any pressure regulation or restriction (“Sample Loop
Pressurization”, Fig. 1). The hydrogen in the loop is then exchange membrane fuel cells (PEMFCs) require high purity
hydrogen for maximum performance. The following are the
released entirely as a “jet pulse” into a T-union which splits
sample into a 0.25 µm ID 30 m long capillary column and an reported effects (SAE TIR J2719) of the compounds deter-
mined by this test method.
electronic flow controller (EFC) used to vent excess hydrogen
to the atmosphere (“Jet Pulse Injection”, Fig. 2). Less than 1%
5.2 Carbon Dioxide (CO ), acts largely as a diluent, how-
of hydrogen enters the capillary column with the remaining
ever in the fuel cell environment CO can be transformed into
sample venting to atmosphere through EFC. As demonstrated
CO.
inAppendixX1,thehydrogenvolume“jetpulseinjected”into
5.3 Water (H O), is an inert impurity, as it does not affect
the capillary column is a constant volume and independent of
the function of a fuel cell stack; however, it provides a
thesamplelooppressurewhenthesamplelooppressureisover
transport mechanism for water-soluble contaminants, such as
90 psi. Therefore, the constant hydrogen volume from stan-
+ +
Na or K . In addition, it may form ice on valve internal
dards or samples is GC/MS analyzed in regardless of standard
surface at cold weather or react exothermally with metal
or sample pressures.
hydride used as hydrogen fuel storage.
4.3 Jet pulse injected volume into the capillary column is
5.4 Inert Gases (N and Ar), do not normally react with a
approximate 100 µL (In Appendix X1, this volume is calcu-
fuel cell components or fuel cell system and are considered
lated to be 115µL under the analytical conditions described in
diluents. Diluents can decrease fuel cell stack performance.
Appendix X1). When a 2-mL of sample loop is pressurized to
200 psi, the hydrogen in the loop is (200 psi/14.7psi)×2mL 5.5 Oxygen (O ), in low concentrations is considered an
or 27 mL. Hence, 99.5% of the hydrogen sample vents to inert impurity, as it does not adversely affect the function of a
atmosphere. This type of “Jet Pulse Injection” has been found fuel cell stack; however, it is a safety concern for vehicle on
acceptable for the analysis of high pressure hydrogen fuel board fuel storage as it can react violently with hydrogen to
sample since the hydrogen volume injected is independent of generate water and heat.
FIG. 1 Sample Loop Pressurization
D7649 − 10 (2017)
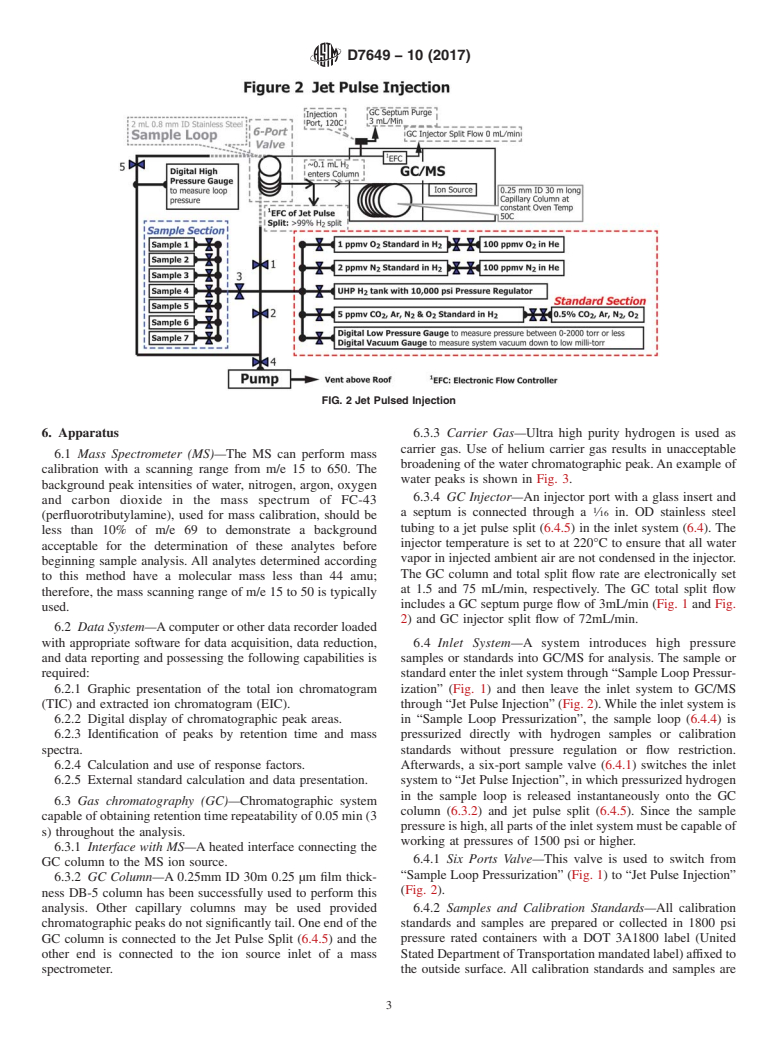
FIG. 2 Jet Pulsed Injection
6. Apparatus 6.3.3 Carrier Gas—Ultra high purity hydrogen is used as
carrier gas. Use of helium carrier gas results in unacceptable
6.1 Mass Spectrometer (MS)—The MS can perform mass
broadening of the water chromatographic peak.An example of
calibration with a scanning range from m/e 15 to 650. The
water peaks is shown in Fig. 3.
background peak intensities of water, nitrogen, argon, oxygen
6.3.4 GC Injector—An injector port with a glass insert and
and carbon dioxide in the mass spectrum of FC-43
a septum is connected through a ⁄16 in. OD stainless steel
(perfluorotributylamine), used for mass calibration, should be
tubing to a jet pulse split (6.4.5) in the inlet system (6.4). The
less than 10% of m/e 69 to demonstrate a background
injector temperature is set to at 220°C to ensure that all water
acceptable for the determination of these analytes before
vapor in injected ambient air are not condensed in the injector.
beginning sample analysis. All analytes determined according
The GC column and total split flow rate are electronically set
to this method have a molecular mass less than 44 amu;
at 1.5 and 75 mL/min, respectively. The GC total split flow
therefore, the mass scanning range of m/e 15 to 50 is typically
includes a GC septum purge flow of 3mL/min (Fig. 1 and Fig.
used.
2) and GC injector split flow of 72mL/min.
6.2 Data System—Acomputer or other data recorder loaded
with appropriate software for data acquisition, data reduction, 6.4 Inlet System—A system introduces high pressure
and data reporting and possessing the following capabilities is samples or standards into GC/MS for analysis. The sample or
required: standard enter the inlet system through “Sample Loop Pressur-
6.2.1 Graphic presentation of the total ion chromatogram ization” (Fig. 1) and then leave the inlet system to GC/MS
(TIC) and extracted ion chromatogram (EIC). through“JetPulseInjection”(Fig.2).Whiletheinletsystemis
6.2.2 Digital display of chromatographic peak areas. in “Sample Loop Pressurization”, the sample loop (6.4.4)is
6.2.3 Identification of peaks by retention time and mass pressurized directly with hydrogen samples or calibration
spectra. standards without pressure regulation or flow restriction.
6.2.4 Calculation and use of response factors. Afterwards, a six-port sample valve (6.4.1) switches the inlet
6.2.5 External standard calculation and data presentation. system to “Jet Pulse Injection”, in which pressurized hydrogen
in the sample loop is released instantaneously onto the GC
6.3 Gas chromatography (GC)—Chromatographic system
column (6.3.2) and jet pulse split (6.4.5). Since the sample
capableofobtainingretentiontimerepeatabilityof0.05min(3
pressureishigh,allpartsoftheinletsystemmustbecapableof
s) throughout the analysis.
working at pressures of 1500 psi or higher.
6.3.1 Interface with MS—Aheated interface connecting the
6.4.1 Six Ports Valve—This valve is used to switch from
GC column to the MS ion source.
“Sample Loop Pressurization” (Fig. 1) to “Jet Pulse Injection”
6.3.2 GC Column—A 0.25mm ID 30m 0.25 µm film thick-
(Fig. 2).
ness DB-5 column has been successfully used to perform this
analysis. Other capillary columns may be used provided 6.4.2 Samples and Calibration Standards—All calibration
chromatographicpeaksdonotsignificantlytail.Oneendofthe standards and samples are prepared or collected in 1800 psi
GC column is connected to the Jet Pulse Split (6.4.5) and the pressure rated containers with a DOT 3A1800 label (United
other end is connected to the ion source inlet of a mass StatedDepartmentofTransportationmandatedlabel)affixedto
spectrometer. the outside surface. All calibration standards and samples are
D7649 − 10 (2017)
FIG. 3 m/e18 Extracted Ion Chromatogram of Sample Analysis with Co-Injection of Ambient Air
connected to the inlet system before beginning an analytic 6.4.6 Digital Vacuum Gauge—capable of measuring abso-
sequencetominimizethepotentialforairormoisturecontami- lute pressure at vacuum range 0 to 12,000 milli-torr (mtorr or
-3
nation due to addition or replacement of standard or sample 10 torr). For the vacuum range from 0 to 1000 mtorr, the
containers. accuracy is 6 10% or6 10 mtorr, whichever is larger.
6.4.3 Vacuum Pump—an oil vacuum pump that can pump 6.4.7 Digital Pressure Gauges—Two types of digital pres-
down to 50 mtorr or less. sure gauges are required. A pressure gauge 0 to 1000 psig is
6.4.4 Sample Loop—stainless steel tubing with ⁄16 in. OD used to measure sample and standard final pressure. Another
and 2 mL inside volume. Both ends of the sample loop are digital pressure gauge in the low and narrow pressure range,
connected to a six port valve (6.4.1). such as 0 to 2000 torr, is used to measure the pressure of pure
6.4.5 Jet Pulse Split—a T-union connects the following gases in initial standard preparation.
three portions. 6.4.8 Pressure Regulator—A 10,000 psi pressure regulator
6.4.5.1 Six port valve (6.4.1) is used to reduce UHP hydrogen pressure to approximate 400
6.4.5.2 Inlet of GC column (6.4.2) psi for calibration standard preparation. It is also used to
6.4.5.3 Inlet of an electronic flow controller (EFC) with its pressurize the inlet system during method blank analysis, and
outlet to ambient air. The flow rate of this EFC is always during inlet system flushing.
electronicallysetat150mL/mintoventmostoftheGCinjector
7. Reference Standards
split flow (72mL/min) during “Sample Loop Pressurization”
(Fig.1)andreleasedhydrogenfrompressurizedsampleloopin 7.1 Typical reference standards are listed in Fig. 1.Two
“Jet Pulse Injection” (Fig. 2). standardspreparedinheliumcontaining100ppmvO and100
D7649 − 10 (2017)
ppmv N , are commercial available. Remaining standards spectrum of FC-43 used for mass calibration. In order to
listed in Fig. 1 are prepared as per below. achieve this condition, the GC column flow rate of GC/MS
system should be set at a high flow rate, such as, 2mL/min,
7.2 0.5% CO,Ar,N and O in hydrogen—An evacuated
2 2 2
while the system is in standby mode to remove any air in the
1-L cylinder is connected to four pressure-regulated com-
carrier gas line. In addition, when any air may be introduced
pressed gas cylinders containing reagent or UHP grade CO ,
into the carrier gas system, such as when changing the
Ar, N and O . The system is evacuated to less than 500 mtorr
2 2
hydrogen carrier gas tank, the GC total split flow rate is set at
with all the regulators opened and the main cylinder valves
100mL/min for an hour to rapidly remove air in the carrier gas
closed. With the system isolated from vac
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: D7649 − 10 D7649 − 10 (Reapproved 2017)
Standard Test Method for
Determination of Trace Carbon Dioxide, Argon, Nitrogen,
Oxygen and Water in Hydrogen Fuel by Jet Pulse Injection
and Gas Chromatography/Mass Spectrometer Analysis
This standard is issued under the fixed designation D7649; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method describes a procedure primarily for the determination of carbon dioxide, argon, nitrogen, oxygen and water
in high pressure fuel cell grade hydrogen by gas chromatograph/mass spectrometer (GC/MS) with injection of sample at the same
pressure as sample without pressure reduction, which is called “Jet Pulse Injection”. The procedures described in this method were
designed to measure carbon dioxide at 0.5micromole per mole (ppmv), Argon 1 ppmv, nitrogen 5 ppmv and oxygen 2 ppmv and
water 4 ppmv.
1.2 The values stated in SI units are standard. The values stated in inch-pound units are for information only.
1.3 The mention of trade names in standard does not constitute endorsement or recommendation for use. Other manufacturers
of equipment or equipment models can be used.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2.1 Other Standards:
SAE TIR J2719 Information Report on the Development of a Hydrogen Quality Guideline for Fuel Cell Vehicles April 2008
3. Terminology
3.1 Definitions of Terms Specific to This Standard:
3.1.1 absolute pressure—pressure measured with reference to absolute zero pressure, usually expressed as kPa, mm Hg, bar or
psi. All the pressures mentioned in this method are absolute pressure.
3.1.2 constituent—A component (or compound) found within a hydrogen fuel mixture.
3.1.3 contaminant—impurity that adversely affects the components within the fuel cell system or the hydrogen storage system
by reacting with its components. An adverse effect can be reversible or irreversible.
3.1.4 dynamic calibration—calibration of an analytical system using calibration gas standard generated by diluting known
concentration compressed gas standards with hydrogen, as used in this method for carbon dioxide, argon, nitrogen and oxygen (7.3
and 7.4).
3.1.5 extracted ion chromatogram (EIC)—a GC/MS chromatogram where a selected ion is plotted to determine the
compound(s) of interest.
3.1.6 fuel cell grade hydrogen—hydrogen satisfying the specifications in SAE TIR J2719.
This test method is under the jurisdiction of ASTM Committee D03 on Gaseous Fuels and is the direct responsibility of Subcommittee D03.14 on Hydrogen and Fuel
Cells.
Current edition approved Dec. 1, 2010April 1, 2017. Published February 2011April 2017. Originally approved in 2010. Last previous edition approved in 2010 as
D7649-10. DOI: 10.1520/D7649–10.10.1520/D7649–10R17.
Available from SAE International (SAE), 400 Commonwealth Dr., Warrendale, PA 15096-0001, http://aerospace.sae.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D7649 − 10 (2017)
3.1.7 hydrogen fuel—hydrogen to be tested without compositional change due to sample introduction, etc.
3.1.8 jet pulse injection—high pressure hydrogen fuel sample is introduced instantaneously at the same pressure into GC/MS.
3.1.9 relative humidity—ratio of actual pressure of existing water vapor to maximum possible pressure of water vapor in the
atmosphere at the same temperature, expressed as a percentage.
3.1.10 response factor (RF)—-the amount in volume (μL) of an analyte divided by the EIC area of the analyte.
3.1.11 static calibration—calibration of an analytical system using standards in a matrix, state or manner different than the
samples to be analyzed, as used in this method for water concentration in hydrogen.
3.2 Acronyms:
3.2.1 FCV—fuel cell vehicle.
3.2.2 PEMFC—proton exchange membrane fuel cell.
4. Summary of Test Method
4.1 The simultaneous analysis of carbon dioxide, argon, nitrogen, oxygen and water at 0.5 – 5 ppmv (micromole per mole) in
hydrogen fuel samples from fueling stations is challenging due to high hydrogen fuel sample pressure and possible contaminations
from ambient air.
4.2 In this method, a small stainless steel loop is initially pressurized with high pressure hydrogen standard or sample without
any pressure regulation or restriction (“Sample Loop Pressurization”, Fig. 1). The hydrogen in the loop is then released entirely
as a “jet pulse” into a T-union which splits sample into a 0.25 μm ID 30 m long capillary column and an electronic flow controller
(EFC) used to vent excess hydrogen to the atmosphere (“Jet Pulse Injection”, Fig. 2). Less than 1% of hydrogen enters the capillary
column with the remaining sample venting to atmosphere through EFC. As demonstrated in Appendix X1, the hydrogen volume
“jet pulse injected” into the capillary column is a constant volume and independent of the sample loop pressure when the sample
loop pressure is over 90 psi. Therefore, the constant hydrogen volume from standards or samples is GC/MS analyzed in regardless
of standard or sample pressures.
4.3 Jet pulse injected volume into the capillary column is approximate 100 μL (In Appendix X1, this volume is calculated to
be 115μL under the analytical conditions described in Appendix X1). When a 2-mL of sample loop is pressurized to 200 psi, the
hydrogen in the loop is (200 psi/14.7psi) × 2 mL or 27 mL. Hence, 99.5% of the hydrogen sample vents to atmosphere. This type
of “Jet Pulse Injection” has been found acceptable for the analysis of high pressure hydrogen fuel sample since the hydrogen
volume injected is independent of the pressures of hydrogen standards or samples. Consequently it is unnecessary to regulate
standards and hydrogen samples to the same pressure. In addition to possible trace leaks or air trapped inside, regulators are not
recommended as moisture on the regulator surface can be released into the sample resulting in a high moisture determination.
4.4 A mass spectrometer provides sensitive and selective detection towards carbon dioxide, argon, nitrogen, oxygen and water.
FIG. 1 Sample Loop Pressurization
D7649 − 10 (2017)
FIG. 2 Jet Pulsed Injection
5. Significance and Use
5.1 Low operating temperature fuel cells such as proton exchange membrane fuel cells (PEMFCs) require high purity hydrogen
for maximum performance. The following are the reported effects (SAE TIR J2719) of the compounds determined by this test
method.
5.2 Carbon Dioxide (CO ), acts largely as a diluent, however in the fuel cell environment CO can be transformed into CO.
2 2
5.3 Water (H O), is an inert impurity, as it does not affect the function of a fuel cell stack; however, it provides a transport
+ +
mechanism for water-soluble contaminants, such as Na or K . In addition, it may form ice on valve internal surface at cold
weather or react exothermally with metal hydride used as hydrogen fuel storage.
5.4 Inert Gases (N and Ar), do not normally react with a fuel cell components or fuel cell system and are considered diluents.
Diluents can decrease fuel cell stack performance.
5.5 Oxygen (O ), in low concentrations is considered an inert impurity, as it does not adversely affect the function of a fuel cell
stack; however, it is a safety concern for vehicle on board fuel storage as it can react violently with hydrogen to generate water
and heat.
6. Apparatus
6.1 Mass Spectrometer (MS)—The MS can perform mass calibration with a scanning range from m/e 15 to 650. The background
peak intensities of water, nitrogen, argon, oxygen and carbon dioxide in the mass spectrum of FC-43 (perfluorotributylamine), used
for mass calibration, should be less than 10% of m/e 69 to demonstrate a background acceptable for the determination of these
analytes before beginning sample analysis. All analytes determined according to this method have a molecular mass less than 44
amu; therefore, the mass scanning range of m/e 15 to 50 is typically used.
6.2 Data System—A computer or other data recorder loaded with appropriate software for data acquisition, data reduction, and
data reporting and possessing the following capabilities is required:
6.2.1 Graphic presentation of the total ion chromatogram (TIC) and extracted ion chromatogram (EIC).
6.2.2 Digital display of chromatographic peak areas.
6.2.3 Identification of peaks by retention time and mass spectra.
6.2.4 Calculation and use of response factors.
6.2.5 External standard calculation and data presentation.
6.3 Gas chromatography (GC)—Chromatographic system capable of obtaining retention time repeatability of 0.05 min (3 s)
throughout the analysis.
6.3.1 Interface with MS—A heated interface connecting the GC column to the MS ion source.
6.3.2 GC Column—A 0.25mm ID 30m 0.25 μm film thickness DB-5 column has been successfully used to perform this analysis.
Other capillary columns may be used provided chromatographic peaks do not significantly tail. One end of the GC column is
connected to the Jet Pulse Split (6.4.5) and the other end is connected to the ion source inlet of a mass spectrometer.
D7649 − 10 (2017)
6.3.3 Carrier Gas—Ultra high purity hydrogen is used as carrier gas. Use of helium carrier gas results in unacceptable
broadening of the water chromatographic peak. An example of water peaks is shown in Fig. 3.
6.3.4 GC Injector—An injector port with a glass insert and a septum is connected through a ⁄16 in. OD stainless steel tubing
to a jet pulse split (6.4.5) in the inlet system (6.4). The injector temperature is set to at 220°C to ensure that all water vapor in
injected ambient air are not condensed in the injector. The GC column and total split flow rate are electronically set at 1.5 and 75
mL/min, respectively. The GC total split flow includes a GC septum purge flow of 3mL/min (Fig. 1 and Fig. 2) and GC injector
split flow of 72mL/min.
6.4 Inlet System—A system introduces high pressure samples or standards into GC/MS for analysis. The sample or standard
enter the inlet system through “Sample Loop Pressurization” (Fig. 1) and then leave the inlet system to GC/MS through “Jet Pulse
Injection” (Fig. 2). While the inlet system is in “Sample Loop Pressurization”, the sample loop (6.4.4) is pressurized directly with
hydrogen samples or calibration standards without pressure regulation or flow restriction. Afterwards, a six-port sample valve
(6.4.1) switches the inlet system to “Jet Pulse Injection”, in which pressurized hydrogen in the sample loop is released
instantaneously onto the GC column (6.3.2) and jet pulse split (6.4.5). Since the sample pressure is high, all parts of the inlet system
must be capable of working at pressures of 1500 psi or higher.
6.4.1 Six Ports Valve—This valve is used to switch from “Sample Loop Pressurization” (Fig. 1) to “Jet Pulse Injection” (Fig.
2).
6.4.2 Samples and Calibration Standards—All calibration standards and samples are prepared or collected in 1800 psi pressure
rated containers with a DOT 3A1800 label (United Stated Department of Transportation mandated label) affixed to the outside
surface. All calibration standards and samples are connected to the inlet system before beginning an analytic sequence to minimize
the potential for air or moisture contamination due to addition or replacement of standard or sample containers.
6.4.3 Vacuum Pump—an oil vacuum pump that can pump down to 50 mtorr or less.
FIG. 3 m/e18 Extracted Ion Chromatogram of Sample Analysis with Co-Injection of Ambient Air
D7649 − 10 (2017)
6.4.4 Sample Loop—stainless steel tubing with ⁄16 in. OD and 2 mL inside volume. Both ends of the sample loop are connected
to a six port valve (6.4.1).
6.4.5 Jet Pulse Split—a T-union connects the following three portions.
6.4.5.1 Six port valve (6.4.1)
6.4.5.2 Inlet of GC column (6.4.2)
6.4.5.3 Inlet of an electronic flow controller (EFC) with its outlet to ambient air. The flow rate of this EFC is always
electronically set at 150mL/min to vent most of the GC injector split flow (72mL/min) during “Sample Loop Pressurization” (Fig.
1) and released hydrogen from pressurized sample loop in “Jet Pulse Injection” (Fig. 2).
-3
6.4.6 Digital Vacuum Gauge—capable of measuring absolute pressure at vacuum range 0 to 12,000 milli-torr (mtorr or 10
torr). For the vacuum range from 0 to 1000 mtorr, the accuracy is 6 10% or6 10 mtorr, whichever is larger.
6.4.7 Digital Pressure Gauges—Two types of digital pressure gauges are required. A pressure gauge 0 to 1000 psig is used to
measure sample and standard final pressure. Another digital pressure gauge in the low and narrow pressure range, such as 0 to 2000
torr, is used to measure the pressure of pure gases in initial standard preparation.
6.4.8 Pressure Regulator—A 10,000 psi pressure regulator is used to reduce UHP hydrogen pressure to approximate 400 psi for
calibration standard preparation. It is also used to pressurize the inlet system during method blank analysis, and during inlet system
flushing.
7. Reference Standards
7.1 Typical reference standards are listed in Fig. 1. Two standards prepared in helium containing 100 ppmv O and 100 ppmv
N , are commercial available. Remaining standards listed in Fig. 1 are prepared as per below.
7.2 0.5% CO , Ar, N and O in hydrogen—An evacuated 1-L cylinder is connected to four pressure-regulated compressed gas
2 2 2
cylinders containing reagent or UHP grade CO , Ar, N and O . The system is evacuated to less than 500 mtorr with all the
2 2 2
regulators opened and the main cylinder valves closed. With the syste
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.