ASTM D3326-07(2024)
(Practice)Standard Practice for Preparation of Samples for Identification of Waterborne Oils
Standard Practice for Preparation of Samples for Identification of Waterborne Oils
SIGNIFICANCE AND USE
4.1 Identification of a recovered oil is determined by comparison with known oils selected because of their possible relationship to the particular recovered oil, for example, suspected or questioned sources. Thus, samples of such known oils must be collected and submitted along with the unknown for analysis. It is unlikely that identification of the sources of an unknown oil by itself can be made without direct matching, that is, solely with a library of analyses.
SCOPE
1.1 This practice covers the preparation for analysis of waterborne oils recovered from water. The identification is based upon the comparison of physical and chemical characteristics of the waterborne oils with oils from suspect sources. These oils may be of petroleum or vegetable/animal origin, or both. Seven procedures are given as follows:
Sections
Procedure A (for samples of more than 50 mL volume containing significant quantities of hydrocarbons with boiling points above 280 °C)
8 to 12
Procedure B (for samples containing significant quantities of hydrocarbons with boiling points above 280 °C)
13 to 17
Procedure C (for waterborne oils containing significant amounts of components boiling below 280 °C and to mixtures of these and higher boiling components)
18 to 22
Procedure D (for samples containing both petroleum and vegetable/animal derived oils)
23 to 27
Procedure E (for samples of light crudes and medium distillate fuels)
28 to 34
Procedure F (for thin films of oil-on-water)
35 to 39
Procedure G (for oil-soaked samples)
40 to 44
1.2 Procedures for the analytical examination of the waterborne oil samples are described in Practice D3415 and Test Methods D3328, D3414, and D3650. Refer to the individual oil identification test methods for the sample preparation method of choice. The deasphalting effects of the sample preparation method should be considered in selecting the best methods.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use. Specific caution statements are given in Sections 6 and 32.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: D3326 − 07 (Reapproved 2024)
Standard Practice for
Preparation of Samples for Identification of Waterborne
Oils
This standard is issued under the fixed designation D3326; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope ization established in the Decision on Principles for the
Development of International Standards, Guides and Recom-
1.1 This practice covers the preparation for analysis of
mendations issued by the World Trade Organization Technical
waterborne oils recovered from water. The identification is
Barriers to Trade (TBT) Committee.
based upon the comparison of physical and chemical charac-
teristics of the waterborne oils with oils from suspect sources.
2. Referenced Documents
These oils may be of petroleum or vegetable/animal origin, or
2.1 ASTM Standards:
both. Seven procedures are given as follows:
D95 Test Method for Water in Petroleum Products and
Sections
Bituminous Materials by Distillation
Procedure A (for samples of more than 50 mL volume
containing significant quantities of hydrocarbons with boiling
D1129 Terminology Relating to Water
points above 280 °C) 8 to 12
D1193 Specification for Reagent Water
Procedure B (for samples containing significant quantities of
hydrocarbons with boiling points above 280 °C) 13 to 17 D3325 Practice for Preservation of Waterborne Oil Samples
Procedure C (for waterborne oils containing significant
D3328 Test Methods for Comparison of Waterborne Petro-
amounts of components boiling below 280 °C and to
leum Oils by Gas Chromatography
mixtures of these and higher boiling components) 18 to 22
Procedure D (for samples containing both petroleum and D3414 Test Method for Comparison of Waterborne Petro-
vegetable/animal derived oils) 23 to 27 3
leum Oils by Infrared Spectroscopy (Withdrawn 2018)
Procedure E (for samples of light crudes and medium distillate
D3415 Practice for Identification of Waterborne Oils
fuels) 28 to 34
Procedure F (for thin films of oil-on-water) 35 to 39
D3650 Test Method for Comparison of Waterborne Petro-
Procedure G (for oil-soaked samples) 40 to 44
leum Oils By Fluorescence Analysis (Withdrawn 2018)
1.2 Procedures for the analytical examination of the water-
D4489 Practices for Sampling of Waterborne Oils
borne oil samples are described in Practice D3415 and Test
E1 Specification for ASTM Liquid-in-Glass Thermometers
Methods D3328, D3414, and D3650. Refer to the individual oil
E133 Specification for Distillation Equipment
identification test methods for the sample preparation method
of choice. The deasphalting effects of the sample preparation 3. Terminology
method should be considered in selecting the best methods.
3.1 Definitions:
1.3 The values stated in SI units are to be regarded as 3.1.1 For definitions of terms used in this standard, refer to
standard. No other units of measurement are included in this Terminology D1129.
standard.
3.2 Definitions of Terms Specific to This Standard:
1.4 This standard does not purport to address all of the
3.2.1 animal/vegetable-derived oils, n—a mixture made of
safety concerns, if any, associated with its use. It is the
mono-, di-, and triglyceride esters of fatty acids and other
responsibility of the user of this standard to establish appro-
substances of animal or vegetable origin, or both.
priate safety, health, and environmental practices and deter-
3.2.2 simulated weathering of waterborne oils by
mine the applicability of regulatory limitations prior to use.
distillation, n—considers only the effect of evaporation, which
Specific caution statements are given in Sections 6 and 32.
likely is the most significant short-term weathering effect in the
1.5 This international standard was developed in accor-
environment.
dance with internationally recognized principles on standard-
1 2
This practice is under the jurisdiction of ASTM Committee D19 on Water and For referenced ASTM standards, visit the ASTM website, www.astm.org, or
is the direct responsibility of Subcommittee D19.06 on Methods for Analysis for contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Organic Substances in Water. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved April 1, 2024. Published April 2024. Originally the ASTM website.
approved in 1974. Last previous edition approved in 2017 as D3326 – 07 (2017). The last approved version of this historical standard is referenced on
DOI: 10.1520/D3326-07R24. www.astm.org.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D3326 − 07 (2024)
3.2.3 simulated weathering of waterborne oils by PROCEDURE A—LARGE SAMPLES
evaporation, n—under ultraviolet light simulates the loss of
8. Scope
light components on weathering, as well as some oxidative
weathering.
8.1 This procedure covers the preparation for analysis of
samples in which the volumes of waterborne oil in the
4. Significance and Use
environmental and suspect source samples equal or exceed
4.1 Identification of a recovered oil is determined by com-
50 mL and in which the oil portion contains significant
parison with known oils selected because of their possible amounts of hydrocarbons with boiling points above 280 °C.
relationship to the particular recovered oil, for example,
NOTE 1—The boiling point may be ascertained by injecting the neat
suspected or questioned sources. Thus, samples of such known
samples into the gas chromatograph and checking the elution times above
oils must be collected and submitted along with the unknown
that of pentadecane on a nonpolar column.
for analysis. It is unlikely that identification of the sources of
8.2 The preparation of samples containing mostly hydrocar-
an unknown oil by itself can be made without direct matching,
bons of boiling points below 280 °C, such as petroleum
that is, solely with a library of analyses.
distillate fuels, is beyond the scope of this procedure (see
Procedure C or E).
5. Reagents and Materials
5.1 Purity of Reagents—Reagent grade chemicals shall be
9. Summary of Procedure
used in all tests. Unless otherwise indicated, it is intended that
9.1 A neat portion of the waterborne oil is retained. If not
all reagents shall conform to the specifications of the Commit-
possible to obtain a neat portion, then retain a portion of the
tee on Analytical Reagents of the American Chemical Society.
waterborne oil as received. This is to be used in those analyses
Special ancillary procedures such as fluorescence may require
performed on samples containing significant quantities of
higher purity grades of solvents. Other grades may be used
hydrocarbons with boiling points below 280 °C. Preparation of
provided it is first ascertained that the reagent is of sufficiently
these samples is beyond the scope of this procedure, but are
high purity to permit its use without lessening the accuracy of
covered in Procedure C.
the determination.
NOTE 2—Waterborne oil samples containing significant quantities of
5.2 Purity of Water—Unless otherwise indicated, references
hydrocarbons with boiling points below 280 °C (see Note 1), such as
to water shall be understood to mean reagent water that meets
gasoline and kerosene, can usually be obtained as neat samples without
the purity specifications of Type I or Type II water, as specified any sample preparation.
in Specification D1193.
9.2 The waterborne oil sample is dissolved in an equal
volume of chloroform or dichloromethane and centrifuged to
6. Caution
remove the free water, solids, and debris. The water layer, if
6.1 Solvents used in this practice are volatile, flammable, or
present, is separated from the organic layer. Other debris, if
may cause the harm to the health of the user. Specifically,
present, is removed by filtration through glass wool.
benzene is a known carcinogen, while chloroform and carbon
NOTE 3—The use of spectrograde cyclohexane is required for the
tetrachloride are suspected carcinogens. Consequently, it is
extraction of samples to be analyzed by fluorescence spectrometry by Test
important that extractions and separations utilizing these sub-
Method D3650. Separation of water may be accomplished by centrifuga-
stances must be carried out in a laboratory hood with a
tion or dying, or both, with anhydrous sodium sulfate.
minimum linear face velocity of 38 m ⁄min to 45 m ⁄min
9.3 When centrifugation will not separate the water from the
(125 ft ⁄min to 150 ft ⁄min) located in a regulated area posted
chloroform solution of the sample, it is refluxed with an
with signs bearing the legends: NO SMOKING or (if appro-
aromatic or petroleum distillate solvent in accordance with Test
priate) DANGER — CHEMICAL CARCINOGEN-
Method D95.
AUTHORIZED PERSONNEL ONLY, or both.
NOTE 4—Pressure filtration has also been found useful for breaking
emulsions.
7. Sampling
9.4 A portion of the solvent/sample solution is retained. The
7.1 Collect representative samples in accordance with Prac-
solvent may be removed by evaporation. This portion of the
tices D4489.
sample may be used in the preliminary gas chromatographic
7.2 Preserve the waterborne oil samples in accordance with
analysis, Test Methods D3328 (Test Method A), and other
Practice D3325.
analyses in which the results are unaffected by weathering.
7.3 The portion of the sample used must be representative of
9.5 The remainder of the solvent/sample solution is distilled
the total sample. If the material is liquid, thoroughly stir the
using nitrogen purge to a liquid temperature of 280 °C to
sample as received, warming if necessary to ensure uniformity.
remove the solvent and simulate weathering conditions as
nearly as possible. The distillate may be discarded or saved for
characterization by gas chromatography (Test Methods
ACS Reagent Chemicals, Specifications and Procedures for Reagents and
Standard-Grade Reference Materials, American Chemical Society, Washington,
D3328). This simulated weathering treatment is necessary to
DC. For suggestions on the testing of reagents not listed by the American Chemical
bring the unweathered suspect samples and the waterborne oil
Society, see Analar Standards for Laboratory Chemicals, BDH Ltd., Poole, Dorset,
sample to as nearly comparable physical condition for subse-
U.K., and the United States Pharmacopeia and National Formulary, U.S. Pharma-
copeial Convention, Inc. (USPC), Rockville, MD. quent analysis as possible. Analyses requiring the use of this
D3326 − 07 (2024)
treated residue include elemental analysis; gas chromato- 10.7 Flowmeter, to regulate flow of nitrogen to distillation
graphic analysis (Test Methods D3328, Test Methods A and B); flask. It should be calibrated and graduated for the range
an infrared procedure (Test Method D3414); a fluorescence test 10 mL ⁄min to 15 mL ⁄min.
method (Test Method D3650); and any applicable test method
11. Reagents and Materials
or practice described in Practice D3415.
11.1 Filter Paper, medium retention, medium fast speed,
NOTE 5—The distillate might yield useful information but is discarded
in this practice. prewashed with solvent used.
11.2 Glass Wool, prewashed with solvent used.
10. Apparatus
11.3 Solvent—Chloroform (stabilized with ethanol) or di-
10.1 Centrifuge, capable of whirling two or more filled
chloromethane is used for dissolution of the waterborne oil
100 mL centrifuge tubes at a speed that is controlled to give a
samples. If water is to be removed by distillation, an aromatic,
relative centrifugal force (rcf) between 500 and 800 at the tip
petroleum distillate, or volatile spirits solvent is required as
of the tubes.
specified in Test Method D95. The safety precautions associ-
ated with the use of the solvent selected should be considered
10.2 Centrifuge Tubes, cone shaped, 100 mL.
before it is used (see Note 3).
10.3 Distillation Apparatus for Water Determination, as
specified in Test Method D95.
12. Procedure
10.4 Distillation Apparatus for Simulated Weathering, as
12.1 Retention of Neat Samples:
described in Specification E133 except fitted with nitrogen-
12.1.1 Decant or siphon off a portion of the neat waterborne
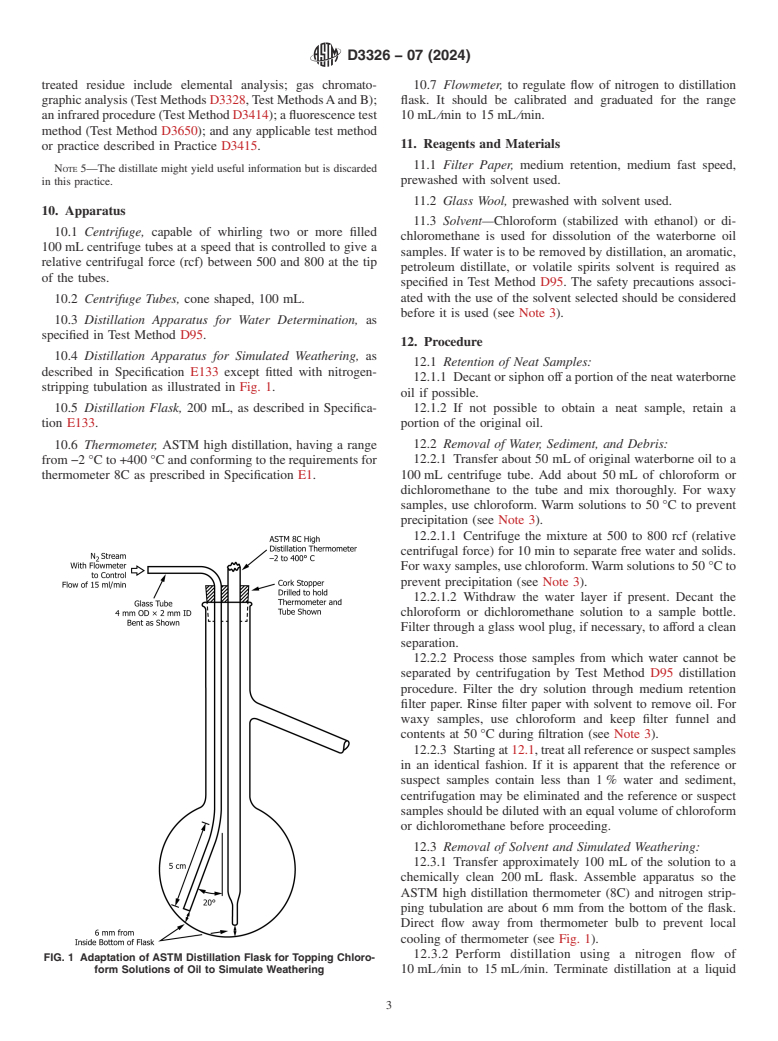
stripping tubulation as illustrated in Fig. 1.
oil if possible.
10.5 Distillation Flask, 200 mL, as described in Specifica- 12.1.2 If not possible to obtain a neat sample, retain a
portion of the original oil.
tion E133.
10.6 Thermometer, ASTM high distillation, having a range 12.2 Removal of Water, Sediment, and Debris:
12.2.1 Transfer about 50 mL of original waterborne oil to a
from −2 °C to +400 °C and conforming to the requirements for
thermometer 8C as prescribed in Specification E1. 100 mL centrifuge tube. Add about 50 mL of chloroform or
dichloromethane to the tube and mix thoroughly. For waxy
samples, use chloroform. Warm solutions to 50 °C to prevent
precipitation (see Note 3).
12.2.1.1 Centrifuge the mixture at 500 to 800 rcf (relative
centrifugal force) for 10 min to separate free water and solids.
For waxy samples, use chloroform. Warm solutions to 50 °C to
prevent precipitation (see Note 3).
12.2.1.2 Withdraw the water layer if present. Decant the
chloroform or dichloromethane solution to a sample bottle.
Filter through a glass wool plug, if necessary, to afford a clean
separation.
12.2.2 Process those samples from which water cannot be
separated by centrifugation by Test Method D95 distillation
procedure. Filter the dry solution through medium retention
filter paper. Rinse filter paper with solvent to remove oil. For
waxy samples, use chloroform and keep filter funnel and
contents at 50 °C during filtration (see Note 3).
12.2.3 Starting at 12.1, treat all reference or suspect samples
in an identical fashion. If it is apparent that the reference or
suspect samples contain less than 1 % water and sediment,
centrifugation may be eliminated and the reference or suspect
samples should be diluted with an equal volume of chloroform
or dichloromethane before proceeding.
12.3 Removal of Solvent and Simulated Weathering:
12.3.1 Transfer approximately 100 mL of the solution to a
chemically clean 200 mL flask. Assemble apparatus so the
ASTM high distillation thermometer (8C) and nitrogen strip-
ping tubulation are about 6 mm from the bottom of the flask.
Direct flow away from thermometer bulb to prevent local
cooling of thermometer (see Fig. 1).
12.3.2 Perform distillation using a nitrogen
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.