ASTM G59-97(2020)
(Test Method)Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements
Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements
SIGNIFICANCE AND USE
3.1 This test method can be utilized to verify the performance of polarization resistance measurement equipment including reference electrodes, electrochemical cells, potentiostats, scan generators, measuring and recording devices. The test method is also useful for training operators in sample preparation and experimental techniques for polarization resistance measurements.
3.2 Polarization resistance can be related to the rate of general corrosion for metals at or near their corrosion potential, Ecorr. Polarization resistance measurements are an accurate and rapid way to measure the general corrosion rate. Real time corrosion monitoring is a common application. The technique can also be used as a way to rank alloys, inhibitors, and so forth in order of resistance to general corrosion.
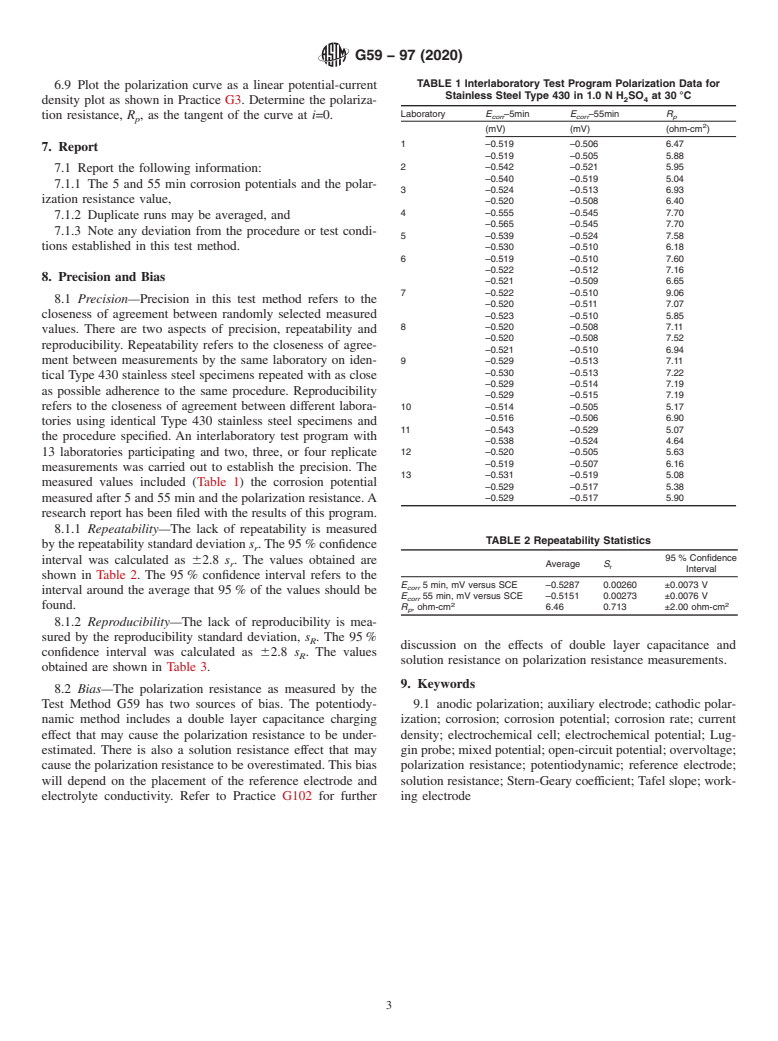
3.3 In this test method, a small potential scan, ΔE(t), defined with respect to the corrosion potential (ΔE = E – Ecorr), is applied to a metal sample. The resultant currents are recorded. The polarization resistance, RP, of a corroding electrode is defined from Eq 1 as the slope of a potential versus current density plot at i = 0 (1-4):3
The current density is given by i. The corrosion current density, icorr, is related to the polarization resistance by the Stern-Geary coefficient, B. (3),
The dimension of Rp is ohm-cm2, icorr is muA/cm2, and B is in V. The Stern-Geary coefficient is related to the anodic, ba, and cathodic, bc, Tafel slopes as per Eq 3.
The units of the Tafel slopes are V. The corrosion rate, CR, in mm per year can be determined from Eq 4 in which EW is the equivalent weight of the corroding species in grams and ρ is the density of the corroding material in g/cm3.
Refer to Practice G102 for derivations of the above equations and methods for estimating Tafel slopes.
3.4 The test method may not be appropriate to measure polarization resistance on all materials or in all environments. See 8.2 for a discussion of method biase...
SCOPE
1.1 This test method covers an experimental procedure for polarization resistance measurements which can be used for the calibration of equipment and verification of experimental technique. The test method can provide reproducible corrosion potentials and potentiodynamic polarization resistance measurements.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: G59 − 97 (Reapproved 2020)
Standard Test Method for
Conducting Potentiodynamic Polarization Resistance
Measurements
This standard is issued under the fixed designation G59; the number immediately following the designation indicates the year of original
adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A superscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3. Significance and Use
3.1 This test method can be utilized to verify the perfor-
1.1 This test method covers an experimental procedure for
mance of polarization resistance measurement equipment in-
polarization resistance measurements which can be used for the
cluding reference electrodes, electrochemical cells,
calibration of equipment and verification of experimental
potentiostats, scan generators, measuring and recording de-
technique. The test method can provide reproducible corrosion
vices. The test method is also useful for training operators in
potentials and potentiodynamic polarization resistance mea-
sample preparation and experimental techniques for polariza-
surements.
tion resistance measurements.
1.2 The values stated in SI units are to be regarded as
3.2 Polarization resistance can be related to the rate of
standard. No other units of measurement are included in this
general corrosion for metals at or near their corrosion potential,
standard.
E . Polarization resistance measurements are an accurate and
corr
1.3 This standard does not purport to address all of the
rapid way to measure the general corrosion rate. Real time
safety concerns, if any, associated with its use. It is the
corrosion monitoring is a common application. The technique
responsibility of the user of this standard to establish appro-
can also be used as a way to rank alloys, inhibitors, and so forth
priate safety, health, and environmental practices and deter-
in order of resistance to general corrosion.
mine the applicability of regulatory limitations prior to use.
3.3 In this test method, a small potential scan, ΔE(t), defined
1.4 This international standard was developed in accor-
with respect to the corrosion potential (ΔE = E – E ), is
corr
dance with internationally recognized principles on standard-
applied to a metal sample. The resultant currents are recorded.
ization established in the Decision on Principles for the
The polarization resistance, R , of a corroding electrode is
P
Development of International Standards, Guides and Recom-
defined from Eq 1 as the slope of a potential versus current
mendations issued by the World Trade Organization Technical
density plot at i = 0 (1-4):
Barriers to Trade (TBT) Committee.
] ΔE
R 5 (1)
S D
p
] i
i50, dE/dt→0
2. Referenced Documents
2 The current density is given by i. The corrosion current
2.1 ASTM Standards:
density, i , is related to the polarization resistance by the
corr
G3 Practice for Conventions Applicable to Electrochemical
Stern-Geary coefficient, B. (3),
Measurements in Corrosion Testing
B
G5 Reference Test Method for Making Potentiodynamic
i 5 10 (2)
corr
R
Anodic Polarization Measurements
p
G102 Practice for Calculation of Corrosion Rates and Re-
2 2
The dimension of R is ohm-cm , i is muA/cm , and B is
p corr
lated Information from Electrochemical Measurements
in V. The Stern-Geary coefficient is related to the anodic, b ,
a
and cathodic, b , Tafel slopes as per Eq 3.
c
b b
a c
B 5 (3)
This test method is under the jurisdiction of ASTM Committee G01 on
2.303 b 1b
~ !
a c
Corrosion of Metals and is the direct responsibility of Subcommittee G01.11 on
Electrochemical Measurements in Corrosion Testing.
The units of the Tafel slopes are V. The corrosion rate, CR,
Current edition approved Nov. 1, 2020. Published November 2020. Originally
in mm per year can be determined from Eq 4 in which EW is
approved in 1978. Last previous edition approved in 2014 as G59 – 97 (2014). DOI:
10.1520/G0059-97R20.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on The boldface numbers in parentheses refer to the list of references at the end of
the ASTM website. this standard.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
G59 − 97 (2020)
the equivalent weight of the corroding species in grams and ρ 5.4 If the observed results are different than expected, the
is the density of the corroding material in g/cm . electrochemical equipment may require calibration or servicing
in accordance with the manufacturer’s guidelines.
i EW
corr
CR5 3.273 10 (4)
ρ
6. Experimental Procedure
Refer to Practice G102 for derivations of the above equa-
6.1 The 1.0 N H SO test solution should be prepared from
2 4
tions and methods for estimating Tafel slopes.
American Chemical Society reagent grade acid and distilled
3.4 The test method may not be appropriate to measure
water as described in Test Method G5. The standard test cell
polarization resistance on all materials or in all environments.
requires 900 mL of test solution. The temperature must be
See 8.2 for a discussion of method biases arising from solution
maintained at 30 °C within 1°.
resistance and electrode capacitance.
6.2 The test cell is purged at 150 cm /min with an oxygen-
free gas such as hydrogen, nitrogen, or argon. The purge is
4. Apparatus
started at least 30 min before specimen immersion. The purge
4.1 The apparatus is described in Test Method G5. It
continues throughout the test.
includes a 1 L round bottom flask modified to permit the
6.3 The working electrode should be prepared as detailed in
addition of inert gas, thermometer, and electrodes. This stan-
Test Method G5. The experiment must commence within 1 h of
dard cell or an equivalent cell can be used. An equivalent cell
preparing the electrode. Preparation includes sequential wet
must be constructed of inert materials and be able to reproduce
polishing with 240 grit and 600 grit SiC paper. Determine the
the standard curve in Test Method G5.
surface area of the specimen to the nearest 0.01 cm and
4.2 A potentiostat capable of varying potential at a constant
subtract for the area under the gasket (typically 0.20 cm to 0.25
scan rate and measuring the current is needed.
cm ).
4.3 A method of recording the varying potential and result-
6.4 Immediately prior to immersion the specimen is
ing current is needed.
degreased with a solvent such as acetone and rinsed with
distilled water. The time delay between rinsing and immersion
5. Test of Electrical Equipment
should be minimal.
5.1 Before the polarization resistance measurement is made,
NOTE 2—Samples of the standard AISI Type 430 stainless steel (UNS
the instrument system (potentiostat, X-Y recorder or data
S45000) used in this test method are available to those wishing to evaluate
acquisition system) must be tested to ensure proper function-
their equipment and test procedure from Metal Samples, P.O. Box 8,
ing. For this purpose, connect the potentiostat to a test
Mumford, AL 36268.
electrical circuit (5). While more complex dummy cells are
6.5 Transfer the test specimen to the test cell and position
sometimes needed in electrochemical studies, the simple resis-
the Luggin probe tip 2 mm to 3 mm from the test electrode
tor shown in Fig. 1 is adequate for the present application.
surface. The tip diameter must be no greater than 1 mm.
5.2 Use R = 10.0 Ω. Set the applied potential on the
6.6 Rec
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: G59 − 97 (Reapproved 2014) G59 − 97 (Reapproved 2020)
Standard Test Method for
Conducting Potentiodynamic Polarization Resistance
Measurements
This standard is issued under the fixed designation G59; the number immediately following the designation indicates the year of original
adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A superscript
epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method covers an experimental procedure for polarization resistance measurements which can be used for the
calibration of equipment and verification of experimental technique. The test method can provide reproducible corrosion potentials
and potentiodynamic polarization resistance measurements.
1.2 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety safety, health, and healthenvironmental practices and determine the
applicability of regulatory limitations prior to use.
1.4 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2.1 ASTM Standards:
G3 Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing
G5 Reference Test Method for Making Potentiodynamic Anodic Polarization Measurements
G102 Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements
3. Significance and Use
3.1 This test method can be utilized to verify the performance of polarization resistance measurement equipment including
reference electrodes, electrochemical cells, potentiostats, scan generators, measuring and recording devices. The test method is also
useful for training operators in sample preparation and experimental techniques for polarization resistance measurements.
3.2 Polarization resistance can be related to the rate of general corrosion for metals at or near their corrosion potential, E .
corr
Polarization resistance measurements are an accurate and rapid way to measure the general corrosion rate. Real time corrosion
monitoring is a common application. The technique can also be used as a way to rank alloys, inhibitors, and so forth in order of
resistance to general corrosion.
This test method is under the jurisdiction of ASTM Committee G01 on Corrosion of Metals and is the direct responsibility of Subcommittee G01.11 on Electrochemical
Measurements in Corrosion Testing.
Current edition approved May 1, 2014Nov. 1, 2020. Published May 2014November 2020. Originally approved in 1978. Last previous edition approved in 20092014 as
G59 -– 97 (2009).(2014). DOI: 10.1520/G0059-97R14.10.1520/G0059-97R20.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’sstandard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
G59 − 97 (2020)
3.3 In this test method, a small potential scan, ΔE(t), defined with respect to the corrosion potential (ΔE = E – E ), is applied
corr
to a metal sample. The resultant currents are recorded. The polarization resistance, R , of a corroding electrode is defined from
P
Eq 1 as the slope of a potential versus current density plot at i = 0 (1-4):
] ΔE
R 5 (1)
S D
p
] i
i50, dE/dt→0
The current density is given by i. The corrosion current density, i , is related to the polarization resistance by the Stern-Geary
corr
coefficient, B.(3),
B
i 5 10 (2)
corr
R
p
2 2
The dimension of R is ohm-cm , i is muA/cm , and B is in V. The Stern-Geary coefficient is related to the anodic, b , and
p corr a
cathodic, b , Tafel slopes as per Eq 3.
c
b b
a c
B 5 (3)
2.303~b 1b !
a c
The units of the Tafel slopes are V. The corrosion rate, CR, in mm per year can be determined from Eq 4 in which EW is the
equivalent weight of the corroding species in grams and ρ is the density of the corroding material in g/cm .
i EW
corr
CR 5 3.27 310 (4)
ρ
Refer to Practice G102 for derivations of the above equations and methods for estimating Tafel slopes.
3.4 The test method may not be appropriate to measure polarization resistance on all materials or in all environments. See 8.2 for
a discussion of method biases arising from solution resistance and electrode capacitance.
4. Apparatus
4.1 The apparatus is described in Test Method G5. It includes a 1 L round bottom flask modified to permit the addition of inert
gas, thermometer, and electrodes. This standard cell or an equivalent cell can be used. An equivalent cell must be constructed of
inert materials and be able to reproduce the standard curve in Test Method G5.
4.2 A potentiostat capable of varying potential at a constant scan rate and measuring the current is needed.
4.3 A method of recording the varying potential and resulting current is needed.
5. Test of Electrical Equipment
5.1 Before the polarization resistance measurement is made, the instrument system (potentiostat, X-Y recorder or data acquisition
system) must be tested to ensure proper functioning. For this purpose, connect the potentiostat to a test electrical circuit (5). While
more complex dummy cells are sometimes needed in electrochemical studies, the simple resistor shown in Fig. 1 is adequate for
the present application.
FIG. 1 Arrangement for Testing of Electrical Equipment (Potentiostat, X-Y Recorder)
The boldface numbers in parentheses refer to the list of references at the end of this standard.
G59 − 97 (2020)
5.2 Use R = 10.0 Ω. Set the applied potential on the potentiostat to E = – 30.0 –30.0 mV and apply the potential. The current should
be 3.0 mA by Ohm’s Law, I = E/R.
NOTE 1—When polarization resistance values are measured for systems with different corrosion currents, the value of R should be chosen to cover the
current range of the actual polarization resistance measurement. Expected corrosion currents in the microampere range require R = 1 to 10 kΩ.
5.3 Record the potentiodynamic polarization curve at a scan rate of 0.6 V/h from ΔE = –30 mV to ΔE = +30 mV and back to ΔE
= –30 mV. The plot should be linear, go through the origin, and have a slope 10 Ω. The curves recorded for the forward and reverse
scans should be identical.
5.4 If the observed results are different than expected, the electrochemical equipment may require calibration or servicing in
accordance with the manufacturer’smanufacturer’s guidelines.
6. Experimental Procedure
6.1 The 1.0 N H SO test solution should be prepared from American Chemical Society reagent grade acid and distilled water as
2 4
described in Test Method G5. The standard test cell requires 900 mL of test solution. The temperature must be maintained at
30°C30 °C within 1°.
6.2 The test cell is purged at 150 cm /min with an oxygen-free gas such as hydrogen, nitrogen, or argon. The purge is started at
least 30 min before specimen immersion. The purge continues throughout the test.
6.3 The working electrode should be prepared as detailed in Test Method G5. The experiment must c
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.