ASTM F2260-03
(Test Method)Standard Test Method for Determining Degree of Deacetylation in Chitosan Salts by Proton Nuclear Magnetic Resonance (1H NMR) Spectroscopy
Standard Test Method for Determining Degree of Deacetylation in Chitosan Salts by Proton Nuclear Magnetic Resonance (<sup>1</sup>H NMR) Spectroscopy
SCOPE
1.1 This test method covers the determination of the degree of deacetylation in chitosan and chitosan salts intended for use in biomedical and pharmaceutical applications as well as in Tissue Engineered Medical Products (TEMPs) by high-resolution proton NMR (1H NMR). A guide for the characterization of chitosan salts has been published as Guide F 2103.
1.2 The test method is applicable for determining the degree of deacetylation (% DA) of chitosan chloride and chitosan glutamate salts and is valid for % DA values from 50 up to and including 99. It is simple, rapid, and suitable for routine use. Knowledge of the degree of deacetylation is important for an understanding of the functionality of chitosan salts in TEMP formulations and applications. This test method will assist end users in choosing the correct chitosan for their particular application. Chitosan salts may have utility in drug delivery applications, as a scaffold or matrix material, and in cell and tissue encapsulation applications.
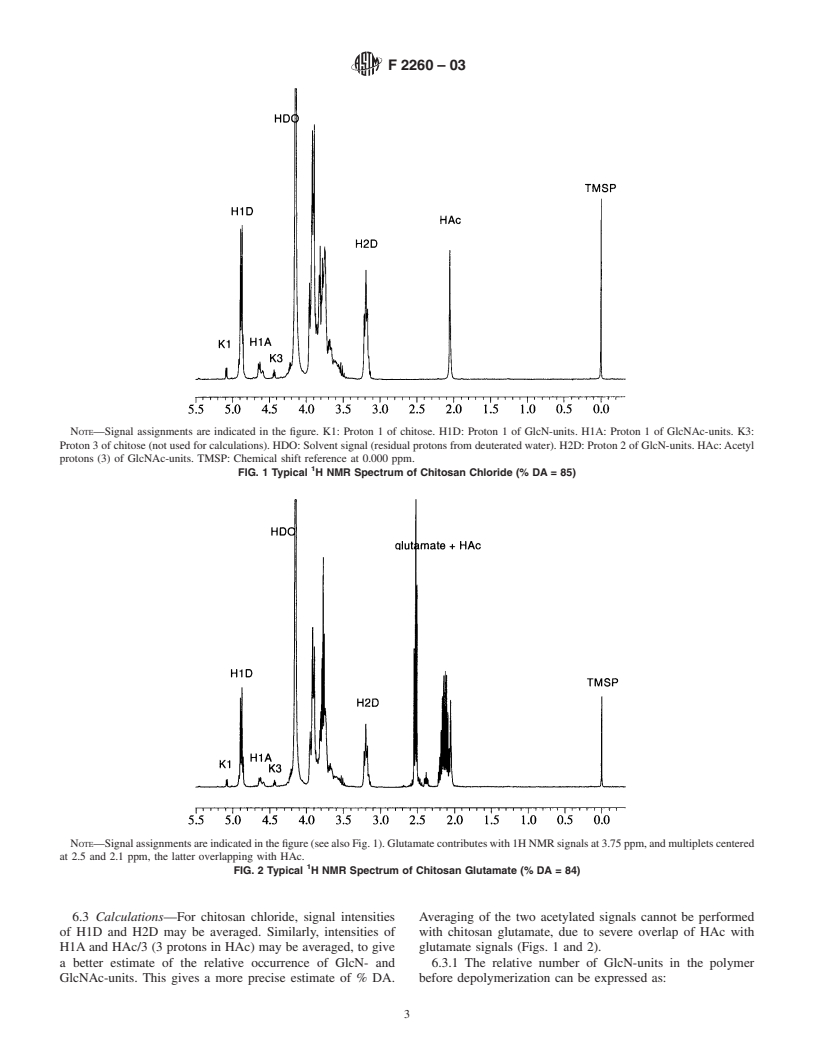
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:F2260–03
Standard Test Method for
Determining Degree of Deacetylation in Chitosan Salts by
Proton Nuclear Magnetic Resonance ( H NMR)
Spectroscopy
This standard is issued under the fixed designation F 2260; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope USP 24-NF19 <761> Nuclear Magnetic Resonance
2.3 European Pharmacopoeia Document:
1.1 This test method covers the determination of the degree
European Pharmacopoeia Monograph 2002:1774 Chitosan
of deacetylation in chitosan and chitosan salts intended for use
Chloride
in biomedical and pharmaceutical applications as well as in
Tissue Engineered Medical Products (TEMPs) by high-
3. Terminology
resolution proton NMR ( H NMR). A guide for the character-
3.1 Definitions:
ization of chitosan salts has been published as Guide F 2103.
3.1.1 chitosan, n—a linear polysaccharide consisting of
1.2 Thetestmethodisapplicablefordeterminingthedegree
b(1→4) linked 2-acetamido-2-deoxy-D-glucopyranose
of deacetylation (% DA) of chitosan chloride and chitosan
(GlcNAc) and 2-amino-2-deoxy-D-glucopyranose (GlcN).
glutamate salts and is valid for % DAvalues from 50 up to and
Chitosan is a polysaccharide derived by N-deacetylation of
including 99. It is simple, rapid, and suitable for routine use.
chitin.
Knowledge of the degree of deacetylation is important for an
3.1.2 degradation, n—change in the chemical structure,
understanding of the functionality of chitosan salts in TEMP
physical properties, or appearance of a material. Degradation
formulations and applications. This test method will assist end
ofpolysaccharidesoccursviacleavageoftheglycosidicbonds.
users in choosing the correct chitosan for their particular
It is important to note that degradation is not synonymous with
application. Chitosan salts may have utility in drug delivery
decomposition. Degradation is often used as a synonym for
applications, as a scaffold or matrix material, and in cell and
depolymerization when referring to polymers.
tissue encapsulation applications.
3.1.3 degree of deacetylation, n—the fraction or percentage
1.3 This standard does not purport to address all of the
of glucosamine units (GlcN: deacetylated monomers) in a
safety concerns, if any, associated with its use. It is the
chitosan polymer molecule.
responsibility of the user of this standard to establish appro-
3.1.4 depolymerization, n—reduction in the length of a
priate safety and health practices and determine the applica-
polymer chain to form shorter polymeric units.
bility of regulatory limitations prior to use.
4. Significance and Use
2. Referenced Documents
4.1 The degree of deacetylation of chitosan salts is an
2.1 ASTM Standards:
important characterization parameter since the charge density
E 386 Practice for Data Presentation Relating to High-
of the chitosan molecule is responsible for potential biological
Resolution Nuclear Magnetic Resonance (NMR) Spectros-
2 and functional effects.
copy
4.2 The degree of deacetylation (% DA) of water-soluble
F 2103 Guide for the Characterization and Testing of Chi-
chitosan salts can be determined by H nuclear magnetic
tosan Salts as Starting Material Intended for Use in
resonance spectroscopy ( H NMR). Several workers have
Biomedical andTissue Engineered Medical ProductAppli-
3 reported on the NMR determination of chemical composition
cations
and sequential arrangement of monomer units in chitin and
2.2 United States Pharmacopeia Document:
This test method is under the jurisdiction ofASTM Committee F04 on Medical
and Surgical Materials and Devices and is the direct responsibility of Subcommittee Available from U.S. Pharmacopeia (USP), 12601 Twinbrook Pkwy., Rockville,
F04.42 on Biomaterials and Biomolecules for TEMPs. MD 20852.
Current edition approved Apr. 10, 2003. Published May 2003. Available from European Directorate for the Quality of Medicines (EDQM),
Annual Book of ASTM Standards, Vol 03.06. Publications and Services, European Pharmacopoeia, BP 907, F-67029 Strasbourg,
Annual Book of ASTM Standards, Vol 13.01. France.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
F2260–03
chitosan. The test method described is primarily based on the 5.2.2 Laboratory shaking device.
work of Vårum et al. (1991), which represents the first 5.2.3 pH meter or pH paper.
publication on routine determination of chemical composition
5.2.4 5 mm NMR tubes.
in chitosans by solution state H NMR spectroscopy. This test
5.2.5 NMR spectrometer (300 MHz field strength or higher
method is applicable for determining the % DA of chitosan
is recommended although analysis at 100 MHz is possible),
chloride and chitosan glutamate salts. It is a simple, rapid, and
with variable temperature option, capable of maintaining 90 6
suitable method for routine use. Quantitative H NMR spec-
1°C sample temperature during analysis, Analog-digital con-
troscopy reports directly on the relative concentration of
version (ADC) with minimum 16 bit is recommended.
chemically distinct protons in the sample, consequently, no
assumptions, calibration curves or calculations other than
6. Procedure
determination of relative signal intensity ratios are necessary.
6.1 Sample Preparation:
4.3 In order to obtain well-resolved NMR spectra, depoly-
6.1.1 Dissolve 33 mg chitosan chloride or 47 mg chitosan
merization of chitosans to a number average degree of poly-
glutamate in 3.3 mL D O by gentle shaking until completely
merization (DP ) of ~15 to 30 is required. This reduces the
n
dissolved.
viscosityandincreasesthemobilityofthemolecules.Although
6.1.2 Add 250 µL of 1 M DCl and shake. Check that the
there are several options for depolymerization of chitosans, the
sample pH* is <2.
most convenient procedure is that of nitrous acid degradation
6.1.3 Add 100 µLfreshly made NaNO solution (10 mg/mL
in deuterated water. The reaction is selective, stoichiometric
in D O).
with respect to GlcN, rapid, and easily controlled (Allan &
6.1.4 Store the sample at room temperature in the dark for 4
Peyron, 1995). The reaction selectively cleaves after a GlcN-
h.
residue, transforming it into 2,5-anhydro-D-mannose (chitose),
6.1.5 Use 0.1 M or 1 M NaOD to adjust the sample to pH*
consequently, depletion of GlcN after depolymerization is
3.8 to 4.2.
expected. On the other hand, the chitose unit displays charac-
1 6.1.6 Transfer 0.7 mL of the sample solution to an NMR
teristic H NMR signals the intensity of which may be
tube.
estimated and utilized in the calculation of % DA, eliminating
6.1.7 Add 5 µL of 0.15 M TMSP for chemical shift
the need for correction factors. Using the intensity of the
referencing.
chitose signals, the number average degree of polymerization
can easily be calculated as a control of the depolymerization.
NOTE 1—For a sample in 100 % D O, the pH reading on a pH meter is
0.4 units lower than the true pD, due to an isotope effect on the glass
4.4 Samples are equilibrated and analyzed at a temperature
electrode. The meter reading in such solvents is normally reported
of 90 6 1°C. Elevated sample temperature contributes to
uncorrected and designated pH*.
reducing sample viscosity and repositions the proton signal of
residualwatertoanareaoutsidethatofinterest.Whilesamples
6.2 Technical Parameters—The most important parameters
are not stored at 90°C but only analyzed at this elevated
used for quantitative H NMR analysis of the degree of
temperature, the NMR tubes should be sealed with a stopper to
deacetylation in chitosan salts are as follows:
avoid any evaporation. At a sample pH* of 3.8-4.3 (see 6.1.5
6.2.1 Acquisition:
below), artifactual deacetylation of the sample does not occur
6.2.1.1 H NMR acquisition should be performed at 90°C
during the short equilibration and analysis time.
with sample spinning at 20 Hz using a standard one-
4.5 Ageneral description of NMR can be found in <761> of
dimensional pulse program.
the USP24-NF19.
Nucleus H
Proton spectral width 10 ppm (approx. −0.5→9.5 ppm)
5. Materials Number of scans 64
Relaxation delay 5 s
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.