ASTM D8309-21
(Guide)Standard Guide for Stability Testing of Cannabis-Based Products
Standard Guide for Stability Testing of Cannabis-Based Products
SIGNIFICANCE AND USE
4.1 Stability testing provides evidence on how the quality and safety of cannabis-based product varies with time under the influence of a variety of environmental factors such as temperature, humidity, and light. The stability testing will also establish a re-test period for the cannabis product or a shelf-life for the cannabis product under recommended storage conditions. Recommended test conditions are based on ICH Q1A.
4.2 The choice of test conditions defined in this guideline is based on an analysis of the effects of climatic conditions in the three regions of the European Commission (EC), Japan and the United States. The mean kinetic temperature in any part of the world can be derived from climatic data, and the world can be divided into four climatic zones, I-IV.
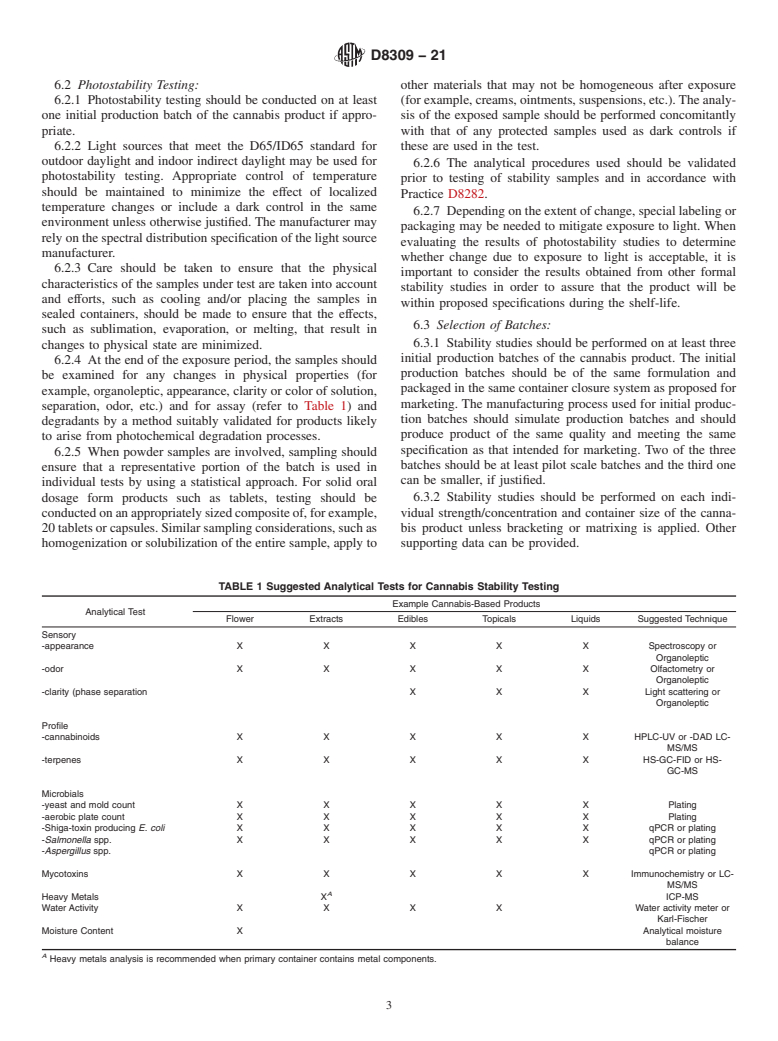
4.3 Requirements of regulatory bodies or governmental departments supersede the recommendations in this guide.
SCOPE
1.1 This guide is applicable to commercial processors and manufacturers engaged in the processing, testing, packaging, labeling, and storage of cannabis products intended for human consumption, including those derived from hemp. Hemp seed and products derived from hemp seed are excluded from the scope of this guide This guide describes the minimum requirements for conducting stability testing of new cannabis products with the purpose of determining appropriate storage conditions and shelf-life.
1.2 This guide applies to all cannabis-derived products commercially manufactured and distributed for consumer use, regardless of the type of cannabis plant from which they were derived.
1.3 Units—The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.5 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: D8309 − 21
Standard Guide for
1
Stability Testing of Cannabis-Based Products
This standard is issued under the fixed designation D8309; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
3
1. Scope 2.2 ICH Documents:
Q1A(R2) Harmonised Tripartite Guideline – Stability Test-
1.1 This guide is applicable to commercial processors and
ing of New Drug Substances and Products
manufacturers engaged in the processing, testing, packaging,
Q1B Harmonised Tripartite Guideline – Stability Testing:
labeling, and storage of cannabis products intended for human
Photostability Testing of New Drug Substances and Prod-
consumption, including those derived from hemp. Hemp seed
ucts
and products derived from hemp seed are excluded from the
scope of this guide This guide describes the minimum require-
3. Terminology
mentsforconductingstabilitytestingofnewcannabisproducts
with the purpose of determining appropriate storage conditions 3.1 Definitions:
and shelf-life.
3.1.1 For definitions of terms, see Terminology D8270.
3.2 Definitions of Terms Specific to This Standard:
1.2 This guide applies to all cannabis-derived products
3.2.1 accelerated testing, n—studies designed to increase
commercially manufactured and distributed for consumer use,
the rate of chemical degradation or physical change of a
regardless of the type of cannabis plant from which they were
product by using exaggerated storage conditions as part of the
derived.
formal stability studies.
1.3 Units—The values stated in SI units are to be regarded
3.2.1.1 Discussion—Data from these studies, in addition to
as standard. No other units of measurement are included in this
long term stability studies, can be used to assess longer term
standard.
chemical effects at non-accelerated conditions and to evaluate
1.4 This standard does not purport to address all of the
the effect of short-term excursions outside the label storage
safety concerns, if any, associated with its use. It is the
conditions (for example, during shipping). Results from accel-
responsibility of the user of this standard to establish appro-
erated testing studies are not always predictive of physical
priate safety, health, and environmental practices and deter-
changes.
mine the applicability of regulatory limitations prior to use.
3.2.2 cannabis product, n—products derived from the can-
1.5 This international standard was developed in accor-
nabis plant (flowers or resins) that are intended for human
dance with internationally recognized principles on standard-
consumption and are packaged and labeled in their final form
ization established in the Decision on Principles for the
for marketing.
Development of International Standards, Guides and Recom-
mendations issued by the World Trade Organization Technical 3.2.3 climatic zones, n—the four zones in the world that are
distinguished by their characteristic prevalent annual climatic
Barriers to Trade (TBT) Committee.
conditions.
2. Referenced Documents
3.2.3.1 Discussion—Zone 1 is temperate, Zone II is
2
2.1 ASTM Standards:
subtropical, Zone III is hot dry, and Zone IV is hot humid/
D8270 Terminology Relating to Cannabis
tropical. This is based on the concept described by Grimm,
4
D8282 Practice for Laboratory Test Method Validation and
1985 and 1986.
Method Development
3.2.4 D65/ID65, n—anilluminantstandardusedtorepresent
daylight as defined by the International Commission on Illu-
mination.
1
This guide is under the jurisdiction ofASTM Committee D37 on Cannabis and
is the direct responsibility of Subcommittee D37.03 on Laboratory.
Current edition approved June 1, 2021. Published July 2021. DOI: 10.1520/
3
D8309-21. Available from International Council for Harmonisation of Technical Require-
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or ments for Pharmaceuticals for Human Use (ICH), ICH Secretariat, Route de
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Pré-Bois, 20, P.O Box 1894, 1215 Geneva, Switzerland, https://www.ich.org.
4
Standards volume information, refer to the standard’s Document Summary page on Grimm, W., Drugs Made in Germany, Vol 28, pp. 196–202, 1985, and Vol 29,
the ASTM website. pp. 39–47, 1986.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
D8309 − 21
3.2.4.1 Discussion—ID65 is the indoo
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.