ASTM D3977-97(2002)
(Test Method)Standard Test Methods for Determining Sediment Concentration in Water Samples
Standard Test Methods for Determining Sediment Concentration in Water Samples
SCOPE
1.1 These test methods cover the determination of sediment concentrations in water and wastewater samples collected from lakes, reservoirs, ponds, streams, and other water bodies. In lakes and other quiescent-water bodies, concentrations of sediment in samples are nearly equal to concentrations at sampling points; in most instances, sample concentrations are not strongly influenced by collection techniques. In rivers and other flowing-water bodies, concentrations of sediment in samples depend upon the manner in which the samples are collected. Concentrations in isokinetically-collected samples can be multiplied by water discharges to obtain sediment discharges in the vicinity of the sampling points.
1.2 The procedures given in these test methods are used by the Agricultural Research Service, Geological Survey, National Resources Conservation Service, Bureau of Reclamation, and other agencies responsible for studying water bodies. These test methods are adapted from a laboratory-procedure manual and a quality-assurance plan.
1.3 These test methods include:SectionsTest Method A-Evaporation8 to 13Test Method B-Filtration14 to 19Test Method C-Wet-sieving-filtration20 to 25
1.4 Test Method A can be used only on sediments that settle within the allotted storage time of the samples which usually ranges from a few days to a few weeks. A correction factor must be applied if dissolved-solids concentration exceeds about 10 % of the sediment concentration.
1.5 Test Method B can be used only on samples containing sand concentrations less than about 10 000 ppm and clay concentrations less than about 200 ppm. The sediment need not be settleable because filters are used to separate water from the sediment. Correction factors for dissolved solids are not required.
1.6 Test Method C can be used if two concentration values are required: one for sand-size particles and one for the combination of silt and clay-size particles. The silt-clay fraction need not be settleable.
1.7 These test methods must not be confused with turbidity measurements discussed in Test Method D 1889. Turbidity is the optical property of a sample that causes light rays to be scattered and absorbed; it is not an accurate measure of the mass or concentration of sediment in the sample.
1.8 These test methods contain some procedures similar to those in Test Methods D 1888 which pertains to measuring particulate and dissolved matter in water.
1.9 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:D 3977–97 (Reapproved 2002)
Standard Test Methods for
Determining Sediment Concentration in Water Samples
This standard is issued under the fixed designation D 3977; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope be settleable because filters are used to separate water from the
sediment. Correction factors for dissolved solids are not
1.1 These test methods cover the determination of sediment
required.
concentrationsinwaterandwastewatersamplescollectedfrom
1.6 Test Method C can be used if two concentration values
lakes, reservoirs, ponds, streams, and other water bodies. In
are required: one for sand-size particles and one for the
lakes and other quiescent-water bodies, concentrations of
combination of silt and clay-size particles. The silt-clay frac-
sediment in samples are nearly equal to concentrations at
tion need not be settleable.
sampling points; in most instances, sample concentrations are
1.7 These test methods must not be confused with turbidity
not strongly influenced by collection techniques. In rivers and
measurements discussed in Test Method D 1889. Turbidity is
other flowing-water bodies, concentrations of sediment in
the optical property of a sample that causes light rays to be
samples depend upon the manner in which the samples are
scattered and absorbed; it is not an accurate measure of the
collected. Concentrations in isokinetically-collected samples
mass or concentration of sediment in the sample.
can be multiplied by water discharges to obtain sediment
1.8 These test methods contain some procedures similar to
discharges in the vicinity of the sampling points.
those in Test Methods D 1888 which pertains to measuring
1.2 The procedures given in these test methods are used by
particulate and dissolved matter in water.
theAgriculturalResearchService,GeologicalSurvey,National
1.9 This standard does not purport to address all of the
Resources Conservation Service, Bureau of Reclamation, and
safety concerns, if any, associated with its use. It is the
other agencies responsible for studying water bodies. These
responsibility of the user of this standard to establish appro-
test methods are adapted from a laboratory-procedure manual
priate safety and health practices and determine the applica-
and a quality-assurance plan.
bility of regulatory limitations prior to use.
1.3 These test methods include:
Sections
2. Referenced Documents
Test Method A—Evaporation 8 to 13
Test Method B—Filtration 14 to 19
2.1 ASTM Standards:
Test Method C—Wet-sieving-filtration 20 to 25
D 1129 Terminology Relating to Water
1.4 Test MethodAcan be used only on sediments that settle D 1193 Specification for Reagent Water
within the allotted storage time of the samples which usually D 1888 Test Methods for Particulate and Dissolved Matter
ranges from a few days to a few weeks. A correction factor in Water
must be applied if dissolved-solids concentration exceeds D 1889 Test Method for Turbidity of Water
about 10 % of the sediment concentration. D 2777 Practice for Determination of Precision and Bias of
1.5 Test Method B can be used only on samples containing Applicable Methods of Committee D-19 on Water
sand concentrations less than about 10 000 ppm and clay D 4410 Terminology for Fluvial Sediment
concentrationslessthanabout200ppm.Thesedimentneednot D 4411 Guide for Sampling Fluvial Sediment in Motion
E11 Specification for Wire-Cloth Sieves for Testing Pur-
poses
These test methods are under the jurisdiction of ASTM Committee D19 on
3. Terminology
Water and are the direct responsibility of Subcommittee D19.07 on Sediments,
Geomorphology, and Open-Channel Flow.
3.1 Definitions—For definitions of water-related terms used
Current edition approved June 10, 2002. Published May 1997. Originally
in these test methods refer to Terminologies D 1129 and
published as D 3977 – 80. Discontinued January 1995 and reinstated as
D 3977 – 97. D 4410.
Guy, H. P., “Laboratory Theory and Methods for Sediment Analysis,” Tech-
niques of Water Resources Investigations, U.S. Geological Survey, Book 5, Chapter
C1, 1941. Annual Book of ASTM Standards, Vol 11.01.
3 5
Matthes,W.J.,Jr.,Sholar,C.,J.,andGeorge,J.R.,“Quality-AssurancePlanfor Discontinued; see 1990 Annual Book of ASTM Standards , Vol 11.01.
the Analysis of Fluvial Sediment,” U.S. Geological Survey Open File Report 90, Annual Book of ASTM Standards, Vol 11.02.
1990. Annual Book of ASTM Standards, Vol 14.02.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
D 3977–97 (2002)
3.2 Definitions of Terms Specific to This Standard: 5. Reagents and Materials
3.2.1 dissolved solids—soluble constituents in water. The
5.1 Purity of Water—Unless otherwise indicated, references
quantityisdeterminedbyevaporatingawatersampletovisible
to water shall be understood to mean reagent water as defined
dryness at a temperature slightly below boiling. The tempera-
by Type III of Specification D 1193.
ture is then raised to 105°C and held for about 2 h. This is
5.1.1 Requirements can usually be met by passing tap water
followed by cooling in a desiccator and weighing the residue.
through a mixed cation-anion exchange resin or by distillation.
3.2.2 fluvial sediment—particles that are (a) derived from
rocks or biological materials and (b) transported by flowing
6. Sampling
water.
6.1 Flows and concentrations in river cross sections are
3.2.3 sediment concentration—(a) the ratio of the mass of
usually unsteady; consequently, in a strict sense, samples
dry sediment in a water-sediment mixture to the mass of the
represent conditions only at the time and location of sample
mixture or (b) the ratio of the mass of dry sediment in a
collection.
water-sediment mixture to the volume of the mixture. As
6.2 A sample may consist of a single container of a
indicated by Table 1, the two ratios differ except at concentra-
water-sediment mixtures collected at (1) a specific point in a
tions less than 8000 mg/L.
river cross section, (2) a specific vertical in a cross section (a
3.2.4 supernate—clear, overlying liquid in a sediment
depth-integrated sample), or (3) several verticals in a cross-
sample.
section. If the verticals are equally spaced and the sample is
3.2.5 suspended sediment—sediment supported by turbu-
collected at equal transit rates, it is referred to as an EWI
lent currents in flowing water or by Brownian movement.
sample.The acronym EWI (equal-width-increment) is synony-
3.2.6 tare—weights of empty containers used in analysis
mous with ETR (equal-transit-rate) which appears in many
procedure.
older reports. A sample may also consist of several containers
filled at different points or verticals in a cross-section. If the
4. Significance and Use
containers are filled at centroids of equal discharge in a cross
section, they are referred to as EDI samples. Details on
4.1 Suspended-sediment samples contain particles with a
sampling are given in Guide D 4411.
widevarietyofphysicalcharacteristics.Bypresentingalternate
approaches, these test methods allow latitude in selecting
7. Sample Handling
analysis methods that work best with the particular samples
under study.
7.1 When samples arrive at the laboratory, group them
4.2 Sediment-concentration data are used for many pur-
according to gaging stations and then arrange each group in
poses that include: (1) computing suspended-sediment dis-
chronological order according to times of sample collection.
charges of streams or sediment yields of watersheds, (2) Separate the samples to be analyzed for concentration from
scheduling treatments of industrial and domestic water sup-
those to be analyzed for particle-size distribution or other
plies, and (3) estimating discharges of pesticides, plant nutri- properties. A data sheet should then be completed for each
ents, and heavy metals transported on surfaces or inside
concentration sample. Examples of three commonly used
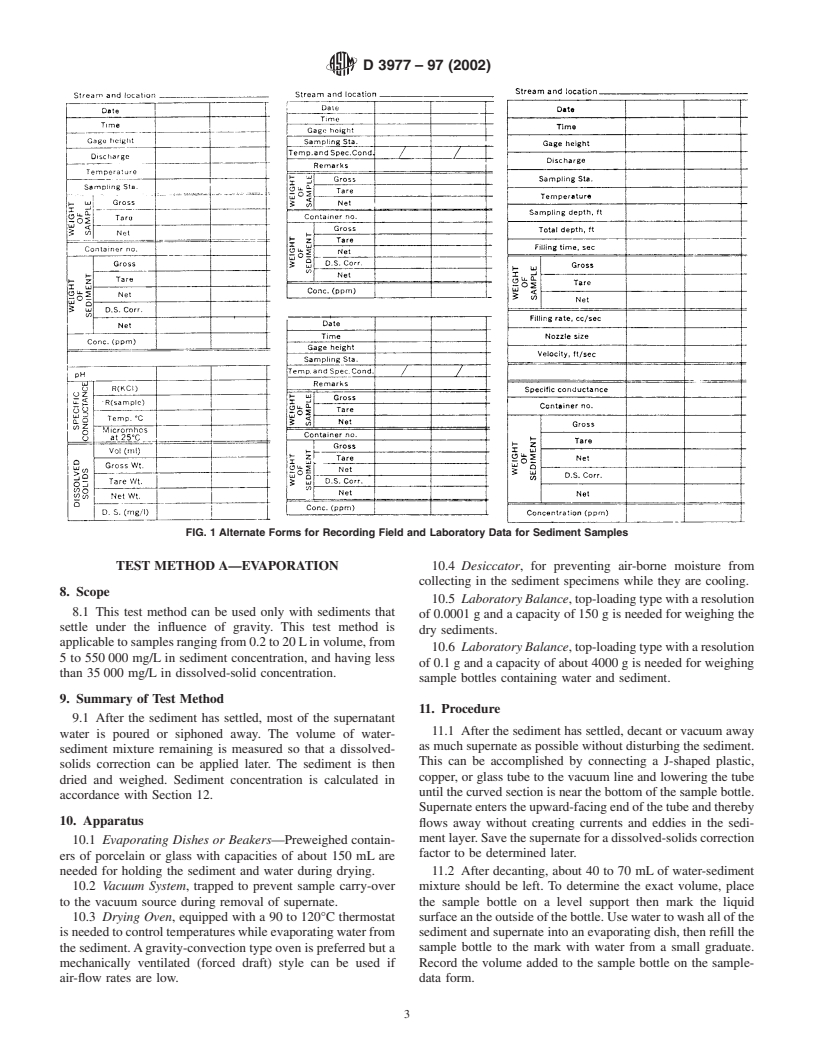
sediment particles. forms are shown on Fig. 1. Expanded notes can be written on
the front of the forms in spaces reserved for other bottles or, if
even more space is needed, remarks can be written on the back
TABLE 1 Factors for Conversion of Sediment Concentration in of the forms along with reference numbers keyed to the
3 A
Parts per Million (ppm) to Grams per Cubic Metre (g/m ) or
appropriate bottles.
Milligrams per Litre (mg/L)
7.2 Check each sample for: (1) loss of water caused by
Range of Range of Range of
Multiply Multiply Multiply leakage or evaporation, (2) loss of sediment which is some-
Concentration, Concentration, Concentration,
By By By
timesrevealedbythepresenceofparticlesontheoutsideofthe
1000 ppm 1000 ppm 1000 ppm
sample bottle, (3) accuracy of sample-identification notes, and
0–7.95 1.00 153–165 1.11 362–380 1.30
8.0–23.7 1.01 166–178 1.12 381–398 1.32 (4) a container tare which is usually etched on the bottle. Enter
23.8–39.1 1.02 179–191 1.13 399–416 1.34
all appropriate notes, observations, and data on the laboratory
39.2–54.3 1.03 192–209 1.14 417–434 1.36
form. Be particularly careful to enter the etched tare reading on
54.4–69.2 1.04 210–233 1.16 435–451 1.38
69.3–83.7 1.05 234–256 1.18 452–467 1.40 the form under the heading Weight of Sample—Tare.
83.8–97.9 1.06 257–278 1.20 468–483 1.42
7.3 Remove the bottle caps then weigh each container along
98.0–111 1.07 279–300 1.22 484–498 1.44
with its water-sediment mixture to the nearest 0.5 g. Record
112–125 1.08 301–321 1.24 499–513 1.46
126–139 1.09 322–341 1.26 514–528 1.48
each reading on the corresponding bottle and on the laboratory
140–152 1.10 342–361 1.28 529–542 1.50
form under the heading Weight of Sample—Gross.
A
Based on water density of 1.000 g/mL and specific gravity of sediment of 2.65.
7.4 Replace the caps then store the samples in a cool, dark
The following equation also applies:
–9
place to minimize microbiological and algal growth. Inspect
C 5 C / 1.0 – C 622 3 10 !
~
the bottles frequently; if the sediment does not settle within
where:
about 14 days, use Test Method B (filtration procedure) for the
C = sediment concentration, mg/L, and
analysis. If settling proceeds at an acceptably rapid rate, use
C = sediment concentration, ppm.
Test Methods A, B, or C.
D 3977–97 (2002)
FIG. 1 Alternate Forms for Recording Field and Laboratory Data for Sediment Samples
TEST METHOD A—EVAPORATION 10.4 Desiccator, for preventing air-borne moisture from
collecting in the sediment specimens while they are cooling.
8. Scope
10.5 Laboratory Balance,top-loadingtypewitharesolution
8.1 This test method can be used only with sediments that
of 0.0001 g and a capacity of 150 g is needed for weighing the
settle under the influence of gravity. This test method is
dry sediments.
applicabletosamplesrangingfrom0.2to20Linvolume,from
10.6 Laboratory Balance,top-loadingtypewitharesolution
5 to 550 000 mg/L in sediment concentration, and having less
of 0.1 g and a capacity of about 4000 g is needed for weighing
than 35 000 mg/L in dissolved-solid concentration.
sample bottles containing water and sediment.
9. Summary of Test Method
11. Procedure
9.1 After the sediment has settled, most of the supernatant
11.1 After the sediment has settled, decant or vacuum away
water is poured or siphoned away. The volume of water-
as much supernate as possible without disturbing the sediment.
sediment mixture remaining is measured so that a dissolved-
This can be accomplished by connecting a J-shaped plastic,
solids correction can be applied later. The sediment is then
copper, or glass tube to the vacuum line and lowering the tube
dried and weighed. Sediment concentration is calculated in
until the curved section is near the bottom of the sample bottle.
accordance with Section 12.
Supernateenterstheupward-facingendofthetubeandthereby
10. Apparatus
flows away without creating currents and eddies in the sedi-
ment layer. Save the supernate for a dissolved-solids correction
10.1 Evaporating Dishes or Beakers—Preweighed contain-
factor to be determined later.
ers of porcelain or glass with capacities of about 150 mL are
needed for holding the sediment and water during drying. 11.2 After decanting, about 40 to 70 mL of water-sediment
10.2 Vacuum System, trapped to prevent sample carry-over mixture should be left. To determine the exact volume, place
to the vacuum source during removal of supernate. the sample bottle on a level support then mark the liquid
10.3 Drying Oven, equipped with a 90 to 120°C thermostat surfaceantheoutsideofthebottle.Usewatertowashallofthe
isneededtocontroltemperatureswhileevaporatingwaterfrom sediment and supernate into an evaporating dish, then refill the
the sediment.Agravity-convection type oven is preferred but a sample bottle to the mark with water from a small graduate.
mechanically ventilated (forced draft) style can be used if Record the volume added to the sample bottle on the sample-
air-flow rates are low. data form.
D 3977–97 (2002)
11.3 Place the evaporating dish in the oven with the 12.4 Modern practice calls for reporting sediment concen-
temperature set slightly below boiling. Maintain this tempera- trations in milligrams per litre instead of ppm as determined in
tureuntilallvisibletracesofwaterhaveevaporated.Thenraise 12.3. Conversion can be made with the aid of Table 1. For
and hold the temperature at 105°C for about 2 h. example, consider a sediment concentration of 41 000 ppm.
11.4 Transferthedishfromtheoventothedesiccator;allow The multiplier obtained from Table 1 is 1.03; therefore, the
the sediment to cool to room temperature. concentration is 41 000 3 1.03 = 42 400 mg/L. The equation
11.5 Weigh the dish to the nearest 0.0001 g as quickly an immediately following Table 1 can be used instead of the
possible to minimize absorption of moisture from the air. multipliers. Eq 1 is easier to use in computer programs and is
Record the weight of the dish and its contents and also the tare applicable to concentrations beyond the range in the table.
weight of the dish on the laboratory form. Subtract the tare
13. Precision and Bias for Test Method A (Evaporation)
from the gross, then record the net weight on the form.
11.6 For nearly all sediment samples, a single drying cycle
13.1 These precision and bias data meet requirements of
is sufficient to obtain stable weight; however, a few samples,
Practice D 2777.
principally those containing high concentrations of or
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.