ASTM F703-96
(Specification)Standard Specification for Implantable Breast Prostheses
Standard Specification for Implantable Breast Prostheses
SCOPE
1.1 This specification covers the requirements for silicone gel filled, saline inflatable silicone gel filled, and saline inflatable, smooth shell implantable breast prostheses intended for use in surgical reconstruction, augmentation, or replacement of the breast.
1.2 Limitations- This specification does not cover custom fabricated implantable breast pprostheses.
1.3 The values stated in SI units are to be regarded as the standard. The inch-pound units given in parentheses are for information only.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: F 703 – 96
Standard Specification for
Implantable Breast Prostheses
This standard is issued under the fixed designation F 703; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 3.1.3 fused or adhered joints (seams)—sites in the shell or
other parts of an implantable breast prosthesis where materials
1.1 This specification covers the requirements for silicone
have been joined (fused or bonded) together, with or without an
gel filled, saline inflatable silicone gel filled, and saline
adhesive, as part of the manufacturing process.
inflatable, smooth-shell implantable breast prostheses intended
3.1.4 gel bleed—diffusion of liquid silicone components of
for use in surgical reconstruction, augmentation, or replace-
silicone gel through the shell of an implantable breast prosthe-
ment of the breast.
sis.
1.2 Limitations— This specification does not cover custom
3.1.5 gel filled breast prosthesis—implantable breast pros-
fabricated implantable breast prostheses.
thesis designed and provided with a pre-filled, fixed volume of
1.3 The values stated in SI units are to be regarded as the
silicone gel.
standard. The inch-pound units given in parentheses are for
3.1.5.1 Type I—a fixed volume gel filled breast prosthesis—
information only.
implantable breast prosthesis comprised of a single lumen
1.4 This standard does not purport to address all of the
containing a fixed amount of silicone gel. The lumen of Type
safety concerns, if any, associated with its use. It is the
I breast prostheses is not accessible for volume adjustments of
responsibility of the user of this standard to establish appro-
any kind.
priate safety and health practices and determine the applica-
3.1.5.2 Type II—double lumen inflatable gel filled breast
bility of regulatory limitations prior to use.
prosthesis—an implantable breast prosthesis comprised of two
2. Referenced Documents complete lumens, one inside the other. The inner lumen
contains a fixed amount of silicone gel and is not accessible for
2.1 ASTM Standards:
volume adjustments of any kind. The outer lumen is provided
D 412 Test Methods for Vulcanized Rubber and Thermo-
with a valve to facilitate filling the void between the inner and
plastic Rubbers and Thermoplastic Elastomers— Tension
outer lumens with saline to adjust the total volume of the
D 1349 Practice for Rubber—Standard Temperatures for
prosthesis, only at the time of use.
Testing
3.1.5.3 Type III—reverse double lumen inflatable gel filled
F 604 Specification for Silicone Elastomers Used in Medi-
breast prosthesis—an implantable breast prosthesis comprised
cal Applications
of two complete lumens, one inside the other. The volume
F 748 Practice for Selecting Generic Biological Test Meth-
between the inner and outer lumens contains a fixed amount of
ods for Materials and Devices
silicone gel and is not accessible for volume adjustments of any
F 1251 Terminology Relating to Polymeric Biomaterials in
kind. The inner lumen is contained within the silicone gel
Medical and Surgical Devices
contained in the outer lumen and has a valve system to
3. Terminology
facilitate filling the inner lumen with saline to increase the
volume of the prosthesis at the time of use. The valve system
3.1 Definitions:
is also designed to facilitate post-operative volume adjustment
3.1.1 barrier coat—a silicone elastomer layer as a part of
by following the instructions provided in product literature.
the shell of a silicone gel implantable breast prostheses that
3.1.6 inflatable breast prosthesis—implantable breast pros-
retards silicone bleed.
theses not containing silicone gel—implantable breast prosthe-
3.1.2 fixation site—an area of the shell of an implantable
ses designed and provided empty and to be filled, all or in part,
breast prosthesis containing material that allows tissue in-
with saline at the time of use to adjust the volume of the
growth.
prosthesis.
3.1.6.1 Type 1—fixed volume inflatable breast prosthesis,an
This specification is under the jurisdiction of ASTM Committee F-4 on Medical
implantable breast prosthesis comprised of a single lumen,
and Surgical Materials and Devicesand is the direct responsibility of Subcommittee
F04.06 on Plastic and Reconstructive Surgery. empty when supplied and having a valve to facilitate filling the
Current edition approved June 10, 1996. Published May 1997. Originally
lumen with the entire volume of saline at the time of use.
published as F 703 – 81. Last previous edition F 703 – 81 (1986).
3.1.6.2 Type II—adjustable inflatable breast prosthesis,an
Annual Book of ASTM Standards, Vol 09.01.
implantable breast prosthesis comprised of a single lumen,
Annual Book of ASTM Standards, Vol 13.01.
Copyright © ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
F 703
empty when supplied and having a valve to facilitate filling the 4.1.4 Vulcanization and Postcure—Time and temperature of
lumen with a system is designed to facilitate further post- vulcanization and postcure must be adjusted with consideration
operative adjustment with saline as instructed in product of the elastomer type and the multi-step fabrication require-
literature. ments of specific prostheses. Final postcure is typically done
3.1.7 low bleed—silicone gel implantable breast prostheses only after the shell or shells and all other portions have been
designed to have minimal silicone bleed when tested by the completely assembled. Time and temperature of final postcure
method in 9.1.1. shall be adequate to drive the chemistry of vulcanization of all
3.1.8 lumen—a cavity within a shell of an implantable elastomers to completion and remove by-products of the cure
breast prosthesis. A lumen may contain either a fixed, non- in keeping with the chemical stoichiometry of the specific cure
adjustable volume of silicone gel, or it may be entirely or partly systems (for example, after postcure no additional vulcaniza-
empty and intended to be inflated (filled) with saline. Inflatable tion should occur when heated additionally at recommended
lumens are accessible by valve to facilitate the addition of cure temperature).
saline to adjust the volume of the prosthesis at the time of use.
4.1.5 Physical Property Testing and Requirements—
More than one lumen may be formed within a shell by silicone
Silicone elastomer shells shall demonstrate an acceptable
elastomer membrane partitions.
response in physical property tests. Prostheses for testing
3.1.9 orientation means—any mark or palpable portion of should be selected from standard production batches which
an implantable breast prosthesis to assist the surgeon in
have gone through all manufacturing processes, including
positioning the implant. sterilization. With silicone gel prostheses, remove gel and clean
3.1.10 saline—only sodium chloride injection USP is rec-
shell with appropriate polar (for example, 2-propanol) or
ommended for filling lumens of implantable breast prosthesis.
non-polar (aliphatic, aromatic, or chlorinated hydrocarbon)
3.1.11 shell—a silicone elastomer continuous layer or mem-
solvent, or both. If solvent cleaned, condition shell afterwards
brane container (sac) that encloses a lumen or multiple lumens
for 3 h 150°F (65.6°C) in an air circulation oven to remove
of an implantable breast prosthesis.
solvent.
3.1.12 silicone elastomer—an elastomer containing cross-
4.1.5.1 Specimen Preparation—Cut required tensile test
linked silicone polymer and fumed amorphous (non-
specimens from shells with Test Methods D 412 dies. Speci-
crystalline) silica as a reinforcing filler.
mens shall be conditioned before testing for at least3hat23
3.1.13 silicone gel—a semisolid material consisting of a
6 2°C (73.4 6 3.6°F).
crosslinked silicone polymer network in which liquid silicone
4.1.6 Test Procedure— Unless otherwise specified, the stan-
polymer is held (see definition of gel in Terminology F 1251).
dard temperature for testing shall be 23 6 2°C (73.4 6 3.6°F).
3.1.14 valve—user sealable or self sealing opening in an
When testing at any other temperature is required, use one of
inflatable or gel saline prosthesis, extending from the exterior
the temperatures specified in Practice D 1349. Requirements
surface of the shell into a lumen, designed to facilitate addition
are as follows:
of saline at the time of use to fill the prosthesis and increase
4.1.6.1 Percent Elongation—Percent elongation shall be
prosthesis volume.
350 % minimum when tested in accordance with Test Methods
D 412.
4. Materials and Manufacture
4.1.6.2 Breaking Strength—Ultimate breaking force in ten-
4.1 Silicone Elastomer—Select and specify elastomers for
sion shall be no less than 11.12 N (2.5 lbs) when tested in
use in implantable breast prostheses in keeping with Specifi-
accordance with Test Methods D 412.
cation F 604.
4.1.6.3 Tensile Set— Determine tensile set at 300 % elon-
4.1.1 Shell—The following describes suitable silicone elas-
gation, stress the specimen for 3 min then allow 3 min for
tomer compositions for use as the primary material of con-
relaxation. The tensile set shall be <10 %, determined in
struction of the shell including the exterior (tissue contact)
accordance with Test Methods D 412.
surface:
4.1.6.4 Critical Fused or Adhered Joints—Joints or seams
Polymer types MQ or VMQ
that are critical to the integrity of the prosthesis envelope shall
Fillers A, B or C
not fail when the shell adjacent to the joint is stressed to 200 %
Additive J (for radiopacity)
Catalysts B, G, J, or K
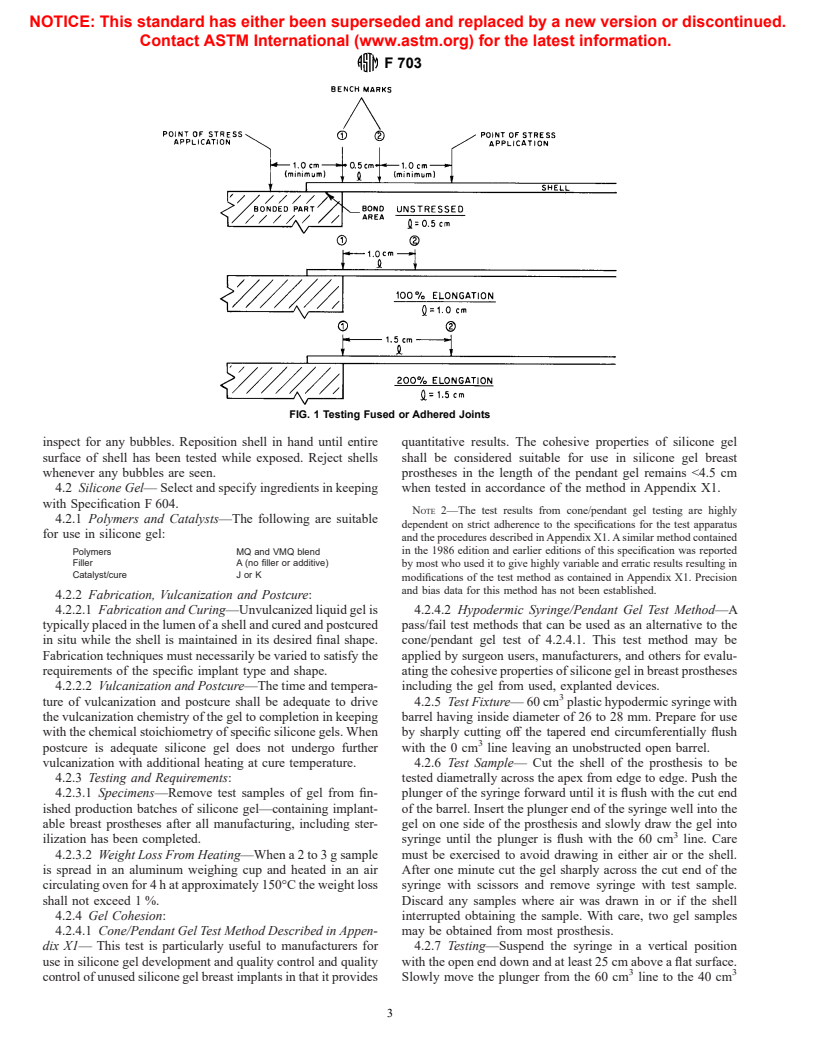
elongation for 10 s (see Fig. 1).
4.1.6.5 Non-Critical Fused or Adhered Joints—Fused joints
4.1.2 Barrier Coatings— The following are suitable com-
or seams that are bonded to the prosthesis envelope but are not
positions of for use in barrier coat elastomers:
critical to the envelope integrity (fixation sites, orientation
Polymer composition FVMQ or VMP M Q
2 2
means, valve covers etc.) shall not fail when the shell adjacent
Fillers B or C
Catalyst/cure J or K
to the joint is stressed to 100 % elongation for 10 s (see Fig. 1).
4.1.7 Shell Rupture/Failure Testing—No standard test for
NOTE 1—The compositions listed in this section are not intended to
limit the composition that may be used providing all other requirements of
assessing shell rupture/failure has yet been developed. When
this specification are satisfied.
such test method has been developed it will be added to this
standard.
4.1.3 Fabrication— Fabrication techniques must necessar-
ily be varied depending on the type of elastomer, the portion of 4.1.8 Shell Leakage Testing—Filla5to8qt stainless steel
an implantable breast prosthesis fabricated, its shape and its bowl with 70 % isopropyl alcohol. Submerge patched shell in
location and function on the prosthesis. bowl and gently apply pressure to the shell assembly. Visually
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
F 703
FIG. 1 Testing Fused or Adhered Joints
inspect for any bubbles. Reposition shell in hand until entire quantitative results. The cohesive properties of silicone gel
surface of shell has been tested while exposed. Reject shells shall be considered suitable for use in silicone gel breast
whenever any bubbles are seen. prostheses in the length of the pendant gel remains <4.5 cm
4.2 Silicone Gel— Select and specify ingredients in keeping when tested in accordance of the method in Appendix X1.
with Specification F 604.
NOTE 2—The test results from cone/pendant gel testing are highly
4.2.1 Polymers and Catalysts—The following are suitable
dependent on strict adherence to the specifications for the test apparatus
for use in silicone gel:
and the procedures described in Appendix X1. A similar method contained
in the 1986 edition and earlier editions of this specification was reported
Polymers MQ and VMQ blend
Filler A (no filler or additive)
by most who used it to give highly variable and erratic results resulting in
Catalyst/cure J or K
modifications of the test method as contained in Appendix X1. Precision
and bias data for this method has not been established.
4.2.2 Fabrication, Vulcanization and Postcure:
4.2.2.1 Fabrication and Curing—Unvulcanized liquid gel is 4.2.4.2 Hypodermic Syringe/Pendant Gel Test Method—A
typically placed in the lumen of a shell and cured and postcured pass/fail test methods that can be used as an alternative to the
in situ while the shell is maintained in its desired final shape. cone/pendant gel test of 4.2.4.1. This test method may be
Fabrication techniques must necessarily be varied to satisfy the applied by surgeon users, manufacturers, and others for evalu-
requirements of the specific implant type and shape. ating the cohesive properties of silicone gel in breast prostheses
4.2.2.2 Vulcanization and Postcure—The time and tempera- including the gel from used, explanted devices.
ture of vulcanization and postcure shall be adequate to drive 4.2.5 Test Fixture—60cm plastic hypodermic syringe with
the vulcanization chemistry of the gel to completion in keeping barrel having inside diameter of 26 to 28 mm. Prepare for use
with the chemical stoichiometry of specific silicone gels. When by sharply cutting off the tapered end circumferentially flush
postcure is adequate silicone gel does not undergo further with the 0 cm line leaving an unobstructed open barrel.
vulcanization with additional heating at cure temperature. 4.2.6 Test Sample— Cut the shell of the prosthesis to be
4.2.3 Testing and Requirements: tested diametrally across the apex from edge to edge. Push the
4.2.3.1 Specimens—Remove test samples of gel from fin- plunger of the syringe forward until it is flush with the cut end
ished production batches of silicone gel—containing implant- of the barrel. Insert the plunger end of the syringe well into the
able breast prostheses after all manufacturing, including ster- gel on one side of the prosthesis and slowly draw the gel into
ilization has been completed. syringe until the plunger is flush with the 60 cm line. Care
4.2.3.2 Weight Loss From Heating—Whena2to3g sample must be exercised to avoid drawing in either air or the shell.
is spread in an aluminum weighing cup and heated in an air After one minute cut the gel sharply across the cut end of the
circulating oven f
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.