ASTM D5085-95
(Test Method)Standard Test Method for Determination of Chloride, Nitrate, and Sulfate in Atmospheric Wet Deposition by Chemically Suppressed Ion Chromatography
Standard Test Method for Determination of Chloride, Nitrate, and Sulfate in Atmospheric Wet Deposition by Chemically Suppressed Ion Chromatography
SCOPE
1.1 This test method is applicable to the determination of chloride, nitrate, and sulfate in atmospheric wet deposition (rain, snow, sleet, and hail) by chemically suppressed ion chromatography (1) using a two pen variable setting recorder and integrator. For additional applications refer to Test Method D4327.
1.2 The concentration ranges for this test method are listed below. The range tested was confirmed using the interlaboratory collaborative test (see Table 1 for statistical summary of the collaborative test). Range of Range Method Tested MDL (mg/L)(2) (mg/L) (mg/L) Chloride 0.03 0.09-2.0 0.15-1.36 Nitrate 0.03 0.09-5.0 0.15-4.92 Sulfate 0.03 0.09-8.0 0.15-6.52
1.3 The method detection limit (MDL) is based on single operator precision (2) and may be higher or lower for other operators and laboratories. The precision and bias data presented are insufficient to justify use at this low level, however, many workers have found that this test method is reliable at lower levels than those that were tested.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. Specific precautionary statements are given in Section 9.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
Designation: D 5085 – 95
Standard Test Method for
Determination of Chloride, Nitrate, and Sulfate in
Atmospheric Wet Deposition by Chemically Suppressed Ion
1,2
Chromatography
This standard is issued under the fixed designation D 5085; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
1.1 This test method is applicable to the determination of 2.1 ASTM Standards:
chloride, nitrate, and sulfate in atmospheric wet deposition D 883 Terminology Relating to Plastics
(rain, snow, sleet, and hail) by chemically suppressed ion D 1129 Terminology Relating to Water
3 5
chromatography (1) using a two pen variable setting recorder D 1193 Specification for Reagent Water
and integrator. For additional applications refer to Test Method D 1356 Terminology Relating to Sampling and Analysis of
D 4327. Atmospheres
1.2 The concentration ranges for this test method are listed D 2777 Practice for Determination of Precision and Bias of
below. The range tested was confirmed using the interlabora- Applicable Methods of Committee D-19 on Water
tory collaborative test (see Table 1 for statistical summary of D 3670 Guide for Determination of Precision and Bias of
the collaborative test). Methods of Committee D-22
D 4210 Practice for Interlaboratory Quality Control Proce-
Range of Range
Method Tested
dures and a Discussion on Reporting Low-Level Data
MDL (mg/L) (2) (mg/L) (mg/L)
D 4327 Test Method for Anions in Water by Chemically
Chloride 0.03 0.09–2.0 0.15–1.36
Suppressed Ion Chromatography
Nitrate 0.03 0.09–5.0 0.15–4.92
Sulfate 0.03 0.09–8.0 0.15–6.52
D 5012 Guide for Preparation of Materials Used for the
Collection and Preservation of Atmospheric Wet Deposi-
1.3 The method detection limit (MDL) is based on single
tion
operator precision (2) and may be higher or lower for other
E 380 Practice for Use of the International System of Units
operators and laboratories. The precision and bias data pre-
(SI) (the Modernized Metric System)
sented are insufficient to justify use at this low level, however,
E 694 Specification for Laboratory Glass Volumetric Appa-
many workers have found that this test method is reliable at
ratus
lower levels than those that were tested.
1.4 This standard does not purport to address all of the
3. Terminology
safety concerns, if any, associated with its use. It is the
3.1 Definitions—For definitions of terms used in this test
responsibility of the user of this standard to establish appro-
method, refer to Terminologies D 883, D 1129, and D 1356 and
priate safety and health practices and determine the applica-
Test Method D 4327 and Practice E 380.
bility of regulatory limitations prior to use. Specific precau-
3.1.1 method detection limit (MDL)—the minimum concen-
tionary statements are given in Section 9.
tration of an analyte that can be reported with 99 % confidence
that the value is above zero based on a standard deviation of
greater than seven repetitive measurements of a solution
This test method is under the jurisdiction of ASTM Committee D-22 on
containing the analyte at a concentration near the low standard.
Sampling and Analysis of Atmospheresand is the direct responsibility of Subcom-
The solution used should not be greater than five times the
mittee D22.06on Atmospheric Deposition.
estimated MDL. (3)
Current edition approved Jan. 15, 1995. Published March 1995. Originally
published as D 5085 – 90. Last previous edition D 5085 – 90.
4. Summary of Test Method
Chemically suppressed ion chromatography is covered by a patent held by
Dionex Corp., 1228 Titan Way, PO Box 3603, Sunnyvale, CA 94088-3603.
4.1 Ion chromatography combines conductometric detection
Interested parties are invited to submit information regarding the identification of
acceptable alternatives to this patented item to the Committee on Standards, ASTM
Headquarters, 100 Barr Harbor Drive, West Conshohocken, PA 19428. Your
comments will receive careful consideration at a meeting of the responsible Annual Book of ASTM Standards, Vol 08.01.
technical committee, that you may attend. Annual Book of ASTM Standards, Vol 11.01.
3 6
The boldface numbers in parentheses refer to references at the end of this test Annual Book of ASTM Standards, Vol 11.03.
method. Annual Book of ASTM Standards, Vol 14.02.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 5085
TABLE 1 Precision and Bias for Chloride, Nitrate, and Sulfate Determined from the Synthetic Atmospheric Wet Deposition Samples
Used in the Interlaboratory Comparison Study
Precision mg/L
Amount Mean
Bias, Significant
A
95 % 95 %
Analyte Added, Recovery, n
B
C D mg/L Bias
S Reproducibility S Repeatability
mg/L mg/L t o
Limit Limit
Chloride 0.15 0.157 36 0.0535 0.150 0.0116 0.0325 0.007 no
0.30 0.293 35 0.0554 0.155 0.0291 0.0815 −0.007 no
0.68 0.652 36 0.0549 0.154 0.0237 0.0664 −0.028 biased low
1.36 1.368 36 0.1 0.28 0.0431 0.121 0.008 no
Nitrate 0.15 0.138 24 0.0362 0.101 0.0289 0.0809 −0.012 no
1.08 1.077 24 0.0495 0.139 0.0421 0.118 −0.003 no
2.44 2.486 22 0.0197 0.0552 0.0183 0.0512 0.046 biased high
4.92 4.999 24 0.126 0.353 0.075 0.21 0.079 biased high
Sulfate 0.15 0.172 36 0.055 0.154 0.0304 0.085 0.022 no
1.43 1.442 35 0.0683 0.191 0.0369 0.103 0.012 no
3.23 3.358 36 0.13 0.364 0.046 0.129 0.128 biased high
6.52 6.775 36 0.37 1.04 0.109 0.305 0.255 biased high
A
Number of samples included in final statistical analysis after removal of outlier data.
B
95 % confidence level.
C
Between laboratory precision, reproducibility.
D
Within laboratory precision (pooled single operator precision), repeatability.
with the separation capabilities of ion exchange resins. (1) A 6.2 Interferences may be caused by ions with retention
filtered aliquot of the sample, ranging in size from 50 to 250 times that are similar to the anion of interest. The retention time
μL, is pumped through an ion exchange column where the of sulfite may be similar to nitrate or sulfate. Other possible
anions of interest are separated. Each ion’s affinity for the interfering ions are bromide and phosphate. Before analyzing
exchange sites, known as its selectivity quotient, is largely precipitation samples, measure the retention times of these
determined by its radius and valence. Because different ions possible interfering ions. Interference is common in some types
have different selectivity quotients, the sample ions elute from of wet deposition samples. If this interference is anticipated,
the column as discrete bands. Each ion is identified by its decreasing the eluent concentration or flow rate, increasing
retention time within the exchange column. The sample ions column length, or decreasing sample size will result in im-
are selectively eluted off the separator column and onto a proved peak resolution.
suppressor column, where the conductivity of the eluent ions is 6.3 Water from the sample injection will cause a negative
reduced and the sample ions are converted to their correspond- peak (water dip) in the chromatogram when it elutes because
ing strong acids. The separated anions are detected by a its conductance is less than that of the suppressed eluent.
conductance cell. The chromatograms produced are displayed Chloride may elute near the water dip and must be sufficiently
on a strip chart recorder or other data acquisition device. resolved from the dip to be accurately quantified. This can be
Measurement of peak height or area is used for quantitation. achieved by changing the eluent concentration or decreasing
The ion chromatograph is calibrated with standard solutions the flow rate. The potential interference of the negative peak
containing known concentrations of the anion(s) of interest. can be eliminated by adding an equivalent of 100 μl of a
Calibration curves are constructed from which the concentra- prepared eluent concentrate (solution that is 100 times more
tion of each analyte in the unknown sample is determined. For concentrated than the eluent used for analysis) per 10.0 mL of
additional information on ion chromatography refer to Test sample. Identical eluent additions must also be included in
Method D 4327. calibration and quality control solutions.
6.4 Decreases in retention times and resolution are symp-
5. Significance and Use
toms of column deterioration which may be caused by the
5.1 This test method is useful for the determination of the
buildup of contaminants on the exchange resin. Refer to the
anions: chloride, nitrate, and sulfate in atmospheric wet depo-
manufacturer’s guidelines for instructions on cleaning the
sition.
column resin and column filter beds. Excising the contami-
5.2 Fig. X1.1 in the appendix represents cumulative fre-
nated portion of the column and changing the filters may also
quency percentile concentration plots of chloride, nitrate, and
improve performance. If the procedure in this section do not
sulfate obtained from analyses of over 5000 wet deposition
restore the retention times, replace the column .
samples. These data may be used as an aid in the selection of
6.5 Contaminated valves and sample lines may also reduce
appropriate calibration solutions. (4)
system performance causing decreased retention times and
resolutions. Refer to the manufacturer’s guidelines for instruc-
6. Interferences
tions on cleaning the valves and replacing the lines.
6.1 Unresolved peaks will result when the concentration of
one of the sample components is 10 to 20 times higher than NOTE 1—Review operational details and refer to the trouble shooting
guide in the Operator’s Manual to determine the cause of decreased
another component that appears in the chromatogram as an
retention times and resolution prior to extensive cleaning or changing of
adjacent peak. Decreasing the eluent concentration or flow rate,
all valves, columns, filters, sample lines, or all of the above.
increasing column length, or decreasing sample size may
correct this problem. 6.6 The presence of air bubbles in the columns, tubing, or
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 5085
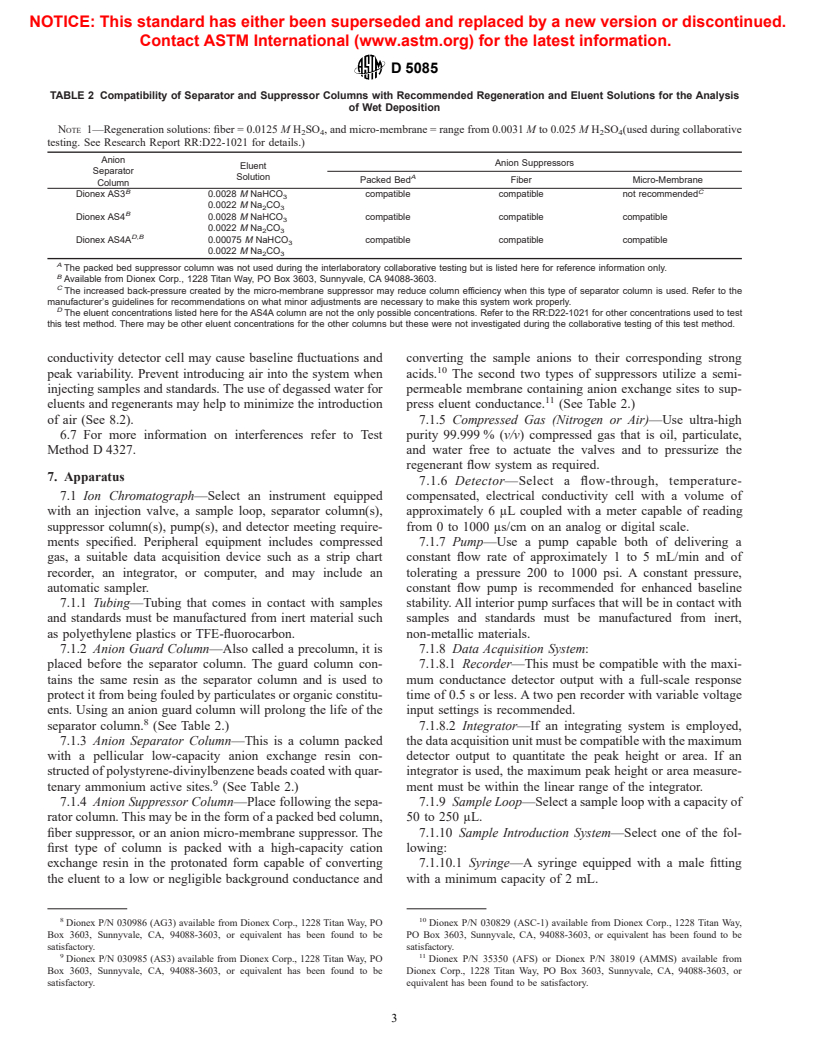
TABLE 2 Compatibility of Separator and Suppressor Columns with Recommended Regeneration and Eluent Solutions for the Analysis
of Wet Deposition
NOTE 1—Regeneration solutions: fiber = 0.0125 M H SO , and micro-membrane = range from 0.0031 M to 0.025 M H SO (used during collaborative
2 4 2 4
testing. See Research Report RR:D22-1021 for details.)
Anion
Anion Suppressors
Eluent
Separator
A
Solution
Packed Bed Fiber Micro-Membrane
Column
B C
Dionex AS3 0.0028 M NaHCO compatible compatible not recommended
0.0022 M Na CO
2 3
B
Dionex AS4 0.0028 M NaHCO compatible compatible compatible
0.0022 M Na CO
2 3
D,B
Dionex AS4A 0.00075 M NaHCO compatible compatible compatible
0.0022 M Na CO
2 3
A
The packed bed suppressor column was not used during the interlaboratory collaborative testing but is listed here for reference information only.
B
Available from Dionex Corp., 1228 Titan Way, PO Box 3603, Sunnyvale, CA 94088-3603.
C
The increased back-pressure created by the micro-membrane suppressor may reduce column efficiency when this type of separator column is used. Refer to the
manufacturer’s guidelines for recommendations on what minor adjustments are necessary to make this system work properly.
D
The eluent concentrations listed here for the AS4A column are not the only possible concentrations. Refer to the RR:D22-1021 for other concentrations used to test
this test method. There may be other eluent concentrations for the other columns but these were not investigated during the collaborative testing of this test method.
conductivity detector cell may cause baseline fluctuations and converting the sample anions to their corresponding strong
peak variability. Prevent introducing air into the system when acids. The second two types of suppressors utilize a semi-
injecting samples and standards. The use of degassed water for permeable membrane containing anion exchange sites to sup-
eluents and regenerants may help to minimize the introduction press eluent conductance. (See Table 2.)
of air (See 8.2). 7.1.5 Compressed Gas (Nitrogen or Air)—Use ultra-high
6.7 For more information on interferences refer to Test purity 99.999 % (v/v) compressed gas that is oil, particulate,
Method D 4327. and water free to actuate the valves and to pressurize the
regenerant flow system as required.
7. Apparatus
7.1.6 Detector—Select a flow-through, temperature-
7.1 Ion Chromatograph—Select an instrument equipped compensated, electrical conductivity cell with a volume of
with an injection valve, a sample loop, separator column(s), approximately 6 μL coupled with a meter capable of reading
suppressor column(s), pump(s), and detector meeting require- from 0 to 1000 μs/cm on an analog or digital scale.
ments specified. Peripheral equipment includes compressed 7.1.7 Pump—Use a pump capable both of delivering a
gas, a suitable data acquisition device such as a strip chart constant flow rate of approximately 1 to 5 mL/min and of
recorder, an integrator, or computer, and may include an tolerating a pressure 200 to 1000 psi. A constant pressure,
automatic sampler. constant flow pump is recommended for enhanced baseline
7.1.1 Tubing—Tubing that comes in contact with samples stability. All interior pump surfaces that will be in contact with
and standards must be manufactured from inert material such samples and standards must be manufactured from inert,
as polyethylene plastics or TFE-fluorocarbon. non-metallic materials.
7.1.2 Anion Guard Column—Also called a precolumn, it is 7.1.8 Data Acquisition System:
placed before the separator column. The guard column con- 7.1.8.1 Recorder—This must be compatible with the maxi-
tains the same resin as the separator column and is used to mum conductance detector output with a full-scale response
protect it from being fouled by particulates or organic constitu- time of 0.5 s or less. A two pen recorder with variable voltage
ents. Using an anion guard column will prolong the life of the input settings is recommended.
separator column. (See Table 2.) 7.1.8.2 Integrator—If an integrating system is employed,
7.1.3 Anion Separato
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.