ASTM D4323-84(1997)e1
(Test Method)Standard Test Method for Hydrogen Sulfide in the Atmosphere by Rate of Change of Reflectance
Standard Test Method for Hydrogen Sulfide in the Atmosphere by Rate of Change of Reflectance
SCOPE
1.1 This test method covers the automatic continuous determination of hydrogen sulfide (H2S) in the atmosphere or in gaseous samples in the range from one part per billion by volume (1 ppb/v) to 3000 ppb/v. Information obtained may be used for air-pollution studies and to monitor for emission sources.
1.2 The range may be extended by appropriate dilution techniques or by equipment modification.
1.3 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use. (See 6.2, 6.3, and 6.4 for specific safety precautionary statements.)
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
e1
Designation: D 4323 – 84 (Reapproved 1997)
Standard Test Method for
Hydrogen Sulfide in the Atmosphere by Rate of Change of
Reflectance
This standard is issued under the fixed designation D 4323; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
e NOTE—Editorial corrections were made throughout in November 1997.
1. Scope proportional to H S concentration.
1.1 This test method covers the automatic continuous deter-
4. Significance and Use
mination of hydrogen sulfide (H S) in the atmosphere or in
4.1 Hydrogen sulfide is an odorous substance which is
gaseous samples in the range from one part per billion by
offensive even at low concentrations in the atmosphere and
volume (1 ppb/v) to 3000 ppb/v. Information obtained may be
toxic at higher levels. It may be a product of biological
used for air-pollution studies and to monitor for emission
processes in the absence of oxygen, as may occur in municipal
sources.
garbage landfills. It is emitted from geothermal sources, occurs
1.2 The range may be extended by appropriate dilution
in oil and gas, and may be emitted from industrial processes.
techniques or by equipment modification.
Measurement is required for air pollution studies, for pollution
1.3 This standard does not purport to address all of the
control, and for plume characterization. Equipment described
safety concerns, if any, associated with its use. It is the
is suitable for fixed site or for mobile monitoring.
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica-
5,6
5. Apparatus
bility of regulatory limitations prior to use. (See 6.2, 6.3, and
5.1 Rate-of-Reaction H S Analyzer—Sample is passed
6.4 for specific safety precautionary statements.)
across a lead acetate-treated surface causing a reflectance
change. Hydrogen sulfide is determined by measuring the rate
2. Referenced Documents
of change of reflectance resulting from darkening when lead
2.1 ASTM Standards:
2 sulfide is formed. Equipment consists of a small flowmeter,
D 1193 Specification for Reagent Water
humidifier, sensing surface exposure chamber, optical system,
D 2420 Test Method for Hydrogen Sulfide in Liquefied
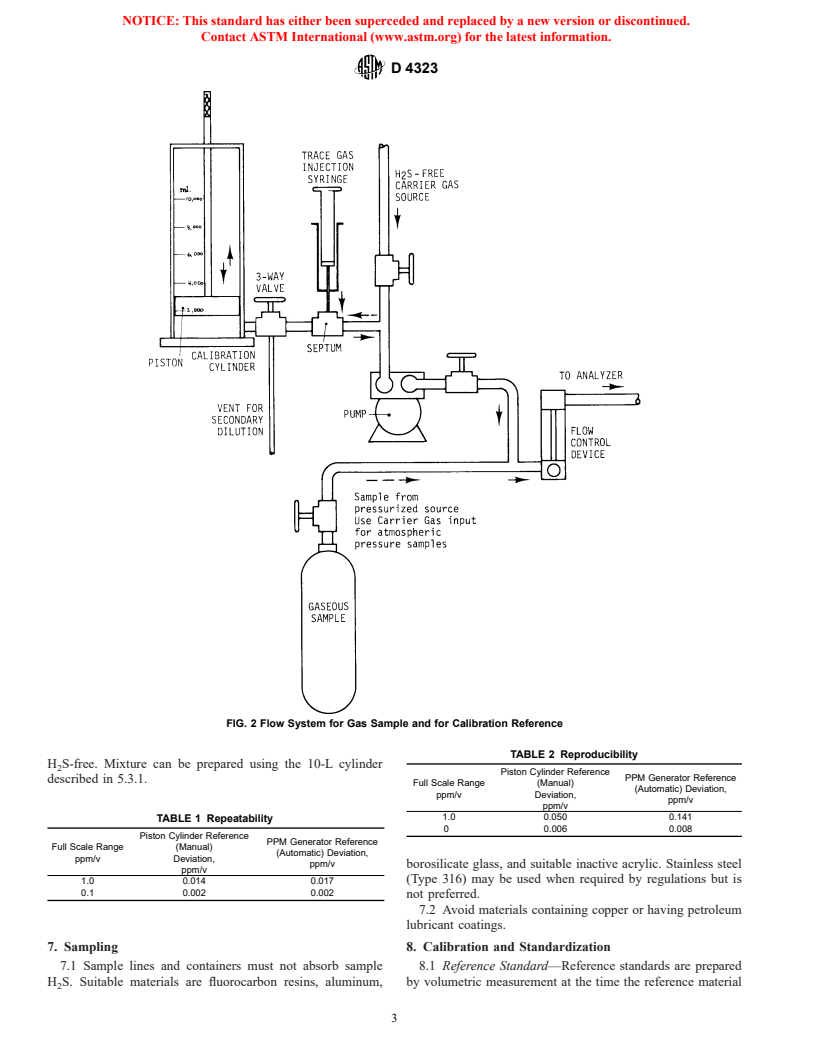
3 and electronic system. (See Fig. 2.) A complete analysis in
Petroleum (LP) Gases (Lead Acetate Method)
about 1 min results from use of the rate of change of color
D 2725 Test Method for Hydrogen Sulfide in Natural Gas
4 rather than magnitude of cumulative color development. The
(Methylene Blue Method)
electronic system provides an output that is proportional to the
3. Summary of Test Method derivative of the photocell signal, caused by reflectance
change, and this rate measurement is a measure of H S
3.1 Hydrogen sulfide is determined by use of the reaction of 2
concentration. A new section of sensing material is drawn into
H S with lead acetate-impregnated paper tape. Detection of the
the sensing chamber at approximately 3-min intervals to
rate of change of reflectance provides measurement in ppb/v
provide a new independent measurement.
ranges with an approximate 3-min analysis cycle time. (See
5.2 Recorder—A method of recording the electronic signal
Fig. 1.) Sample gas is passed through a flowmeter and a
is required. This may take any form that is suitable for the
humidifier; then across lead acetate-treated paper tape. A
record required. A typical system recorder will accept a range
constant humidity is required for a constant reaction rate of
from 0 to 10 V from an output impedance of 1000 V
H S with lead acetate. The resultant change in reflectance is
(maximum). An attenuator or amplifier (usually incorporated
detected by a photocell. The rate of change of reflectance is
1 5
This test method is under the jurisdiction of ASTM Committee D-22 on The sole source of supply of the apparatus described in 5.1, 5.3, and 6.3 known
Sampling and Analysis of Atmospheres and is the direct responsibility of Subcom- to the committee at this time is Houston Atlas, Inc., 22001 N. Park Dr., Houston, TX
mittee D22.03 on Ambient Atmospheres. 77339-3809. If you are aware of alternate suppliers, please provide this informa-
Current edition approved March 1, 1984. Published May 1984. tiohn to ASTM Headquarters. Your comments will receive careful consideration at
2 1
Annual Book of ASTM Standards, Vol 11.01. a meeting of the responsible technical committee , which you may attend.
3 6
Annual Book of ASTM Standards, Vol 05.01. Kimbell, C. L. and Drudhel, H. V., “Trace Sulphur Determination in Petroleum
Annual Book of ASTM Standards, Vol 05.05. Fractions,” Analytical Chemistry, Vol 50, 1978, p 26.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959, United States.
NOTICE: This standard has either been superceded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
D 4323
FIG. 1 Rate of Change of Reflectance Type H S Readout System
into the recorder) may be used for other sensor signal levels. A specifications are available.
chart speed of 1 cm/min is suitable for short term analyses. A 6.2 Acetic Acid Solution (50 mL/L)—Dilute 50 mL of
chart speed of 1 to 5 cm/h is preferable for long-term sampling.
glacial acetic acid (CH COOH), reagent grade, to make 1 L of
Electronic processing, such as integrators, may be added when solution using Type III water prepared as described in Speci-
concentration averages over an interval of time are desirable.
fication D 1193. Caution: Concentrated acetic acid fumes are
an irritant and can cause damage to skin and mucus membrane.
5.3 Reference Gas Preparation:
Handle carefully to avoid injury.
5.3.1 Mixing—A calibrated 10-L cylinder having a movable
6.3 Sensing Tape—Prepare sensing tape as described in Test
piston for use in making volumetric mixtures of gases in the
Method D 2420 or use commercial sensing tape that has been
ppb/v range may be used. Materials of construction must be
prepared in a similar manner. Keep sensing tape in a sealed
inert to H S and not lead to a deterioration of prepared samples.
container to prevent exposure to ambient H S. Caution: Lead
A cylinder of acrylic lubricated with silicone grease and using
acetate is a cumulate poison; wash hands after handling and do
a silicone O-ring has been found to be suitable. Concentration
not breathe any dust containing lead acetate.
remains stable to within 1 % over a 1-h period.
6.4 Hydrogen Sulfide (99.5 %)—Commercially available
5.3.2 Hypodermic Syringe—Gas-tight syringes of 10 and
H S has been found not to be sufficiently pure. Purity certifi-
50-μl capacity. A side port is convenient for purging. Avoid
cation is recommended or a commercially available H S
Luer tip syringes made of plated brass as H S reacts with brass.
generator may be used. Caution: Hydrogen sulfide is toxic at
Other convenient small volume measurement devices such as a
levels above 10 000 ppb/v. Use only under an appropriate
microlitre valve may be used. fume hood. Use protective glasses if liquid H S in cylinders is
handled. Sense of smell may be lost on exposure to H S and is
5.3.3 Pump—A sample pump capable of providing 500 2
unreliable as a warning of danger. (See Appendix X1.1 and
mL/min flow at approximately 35 kPa (5 psi). The pump
X1.2 on Interferences.)
wetted parts must be inert to H S and not lead to a deterioration
6.5 Dilution Gas—A gas similar to the gas to be sampled,
of the sample.
6. Reagent and Materials
Reagent Chemicals, American Chemical Society Specifications, American
6.1 Purity of Reagents—Reagent grade chemicals shall be
Chemical Society, Washington, DC. For suggestions on the testing of reagents not
used in all tests. Unless otherwise noted, all reagents shall
listed by the American Chemical Society, see Analar Standards for Laboratory
conform to the specifications of the Committee on Analytical Chemicals, BDH Ltd., Poole, Dorset, U.K., and the United States Pharmacopeia
and National Formulary, U.S. Pharmaceutical Convention, Inc. (USPC), Rockville,
Reagents of the American Chemical Society, where such
MD.
NOTICE: This standard has eith
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.