ASTM D2621-87(1995)e1
(Test Method)Standard Test Method for Infrared Identification of Vehicle Solids From Solvent-Reducible Paints
Standard Test Method for Infrared Identification of Vehicle Solids From Solvent-Reducible Paints
SCOPE
1.1 This test method covers the qualitative characterization or identification of separated paint vehicle solids by infrared spectroscopy within the limitations of infrared spectroscopy.
1.2 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or discontinued.
Contact ASTM International (www.astm.org) for the latest information.
e1
Designation: D 2621 – 87 (Reapproved 1995)
AMERICAN SOCIETY FOR TESTING AND MATERIALS
100 Barr Harbor Dr., West Conshohocken, PA 19428
Reprinted from the Annual Book of ASTM Standards. Copyright ASTM
Standard Test Method for
Infrared Identification of Vehicle Solids From Solvent-
Reducible Paints
This standard is issued under the fixed designation D 2621; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (e) indicates an editorial change since the last revision or reapproval.
e NOTE—Keywords were added editorially in May 1995.
1. Scope useful for characterizing unknown or competitive coatings, for
complaint investigations, and for in-process control.
1.1 This test method covers the qualitative characterization
or identification of separated paint vehicle solids by infrared
6. Apparatus
spectroscopy within the limitations of infrared spectroscopy.
6.1 Spectrophotometer—A recording double-beam infrared
1.2 This standard does not purport to address all of the
spectrophotometer with a wavelength range from at least 2.5 to
safety concerns, if any, associated with its use. It is the
15 μm and a spectral resolution of at least 0.04 μm over that
responsibility of the user of this standard to establish appro-
range. See Practice E 275.
priate safety and health practices and determine the applica-
6.2 Demountable Cell Mount with NaCl window.
bility of regulatory limitations prior to use.
6.3 Vacuum Drying Oven thermostatically controlled to
2. Referenced Documents operate at 60 6 2°C. A water aspirator vacuum source is
satisfactory.
2.1 ASTM Standards:
6.4 Oven, Gravity or Forced Draft, capable of maintaining
D 1467 Guide for Testing Fatty Acids Used in Protective
temperature from 105 to 110°C.
Coatings
D 1962 Test Method for Saponification Value of Drying
7. Procedure
Oils, Fatty Acids, and Polymerized Fatty Acids
7.1 Place the vehicle, separated from the paint in accordance
D 2372 Practice for Separation of Vehicle from Solvent-
3 with Practice D 2372, on a NaCl window and spread to form a
Reducible Paints
uniform film. Make sure that the thickness of the film is such
E 131 Terminology Relating to Molecular Spectroscopy
that when the infrared spectrum is recorded the transmittance
E 275 Practice for Describing and Measuring Performance
of the strongest band falls between 5 and 15 % (Note). Dry the
of Ultraviolet, Visible, and Near Infared Spectrophotom-
4 film in an oven at 105 to 110°C for 15 min and cool in a
eters
desiccator. Inspect the film visually for defects such as bubbles,
3. Terminology wrinkles, contamination, etc. If defects are present, cast an-
other film. If easily oxidizable substances are present such as
3.1 Definitions:
tung, oiticica, or linseed oils, make sure that the film is dried at
3.1.1 For definitions of terms of symbols, refer to Termi-
60 6 2°C in a vacuum oven for 1 h. If solvents of low volatility
nology E 131.
such as cyclohexanone or isophorone are present, the film may
4. Summary of Test Method need to be dried for several hours in a 60°C vacuum oven.
4.1 Infrared spectra are prepared from dried films of isolated
NOTE 1—Numerous procedures and variations may be used to obtain a
paint vehicles. Vehicle types are identified by comparing the
film on which to prepare a suitable spectrum. These include liquid
spectra to a collection of reference infrared spectra. mounting between two NaCl plates, transmission through free films, and
reflectance from highly polished surfaces.
5. Significance and Use
7.2 Immediately record the infrared spectrum from 2.5 to 15
5.1 The ability to qualitatively identify paint vehicles is
μm so that a spectral resolution of 0.04 μm is maintained
throughout that range (methods for achieving this resolution
will vary according to the directions of the manufacturer of the
This test method is under the jurisdiction of ASTM Committee D-1 on Paint
and Related Coatings, Materials, and Applications and is the direct responsibility of
instrument used).
Subcommittee D01.21 on Chemical Analysis of Paints and Paint Materials.
7.3 Compare the spectrum obtained with reference spectra
Current edition approved June 26, 1987. Published August 1987. Originally
e1
prepared from nonvolatile vehicles of known composition (see
published as D2621 – 67 T. Last previous edition D2621 – 69 (1981) .
Annual Book of ASTM Standards, Vol 06.03.
Annex A1) or consult other published spectra available in the
Annual Book of ASTM Standards, Vol 06.01.
literature (Annex A3). Interpret the spectrum on the basis of
Annual Book of ASTM Standards, Vol 03.06.
D 2621
available information, recognizing certain limitations of infra- 8. Keywords
red spectroscopy, and qualifying the interpretation accordingly
8.1 infrared spectra; paint binders; solvent reducible paint
(Annex A2). Table 1
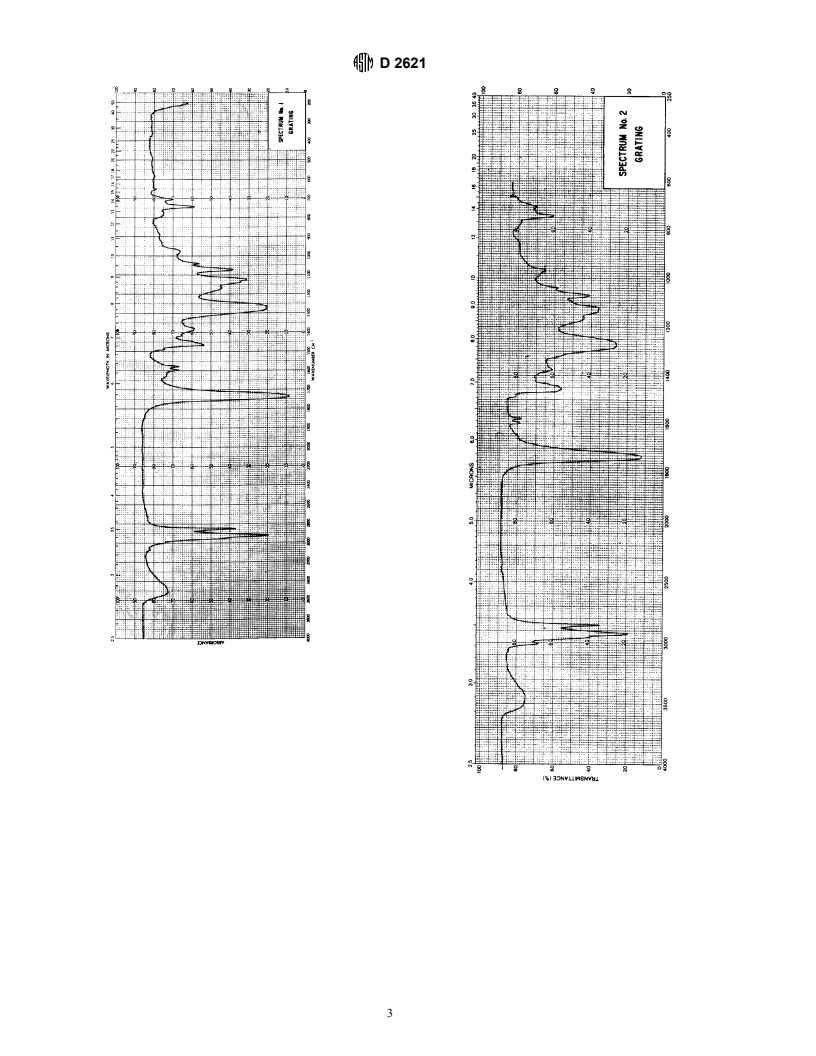
TABLE 1 Correlation of Absorption Bands in Alkyd Spectra
−1
Wavelength, μm Wavenumbers, cm Group Vibration
2.9 3448 O–H stretch
3.4 to 3.5 2941 to 2857 alkane C–H stretch
5.8 1724 ester, C5O stretch
6.2, 6.3, 6.6, 6.7 1613, 1587, 1515, 1493 skeletal in-plane aromatic C5C
6.9, 7.3 1449, 1369 aliphatic C–H bending
7.5 to 9.4 1333 to 1063 ester, C–O–C stretch (o-phthalate ester)
8.6 1163 ester, C–O–C stretch (fatty acid ester)
9.6, 13.5, 14.3 1042, 741, 699 out-of-plane aromatic C–H bending denoting o-disubstituted benzene ring.
ANNEXES
(Mandatory Information)
A1. INFRARED SPECTRA OF NONVOLATILE VEHICLES OF KNOWN COMPOSITION
A1.1 A set of reference infrared spectra on grating and
prism is reproduced on the following pages.
D 2621
D 2621
D 2621
D 2621
D 2621
D 2621
D 2621
D 2621
D 2621
D 2621
D 2621
D 2621
D 2621
D 2621
D 2621
D 2621
D 2621
D 2621
D 2621
D 2621
D 2621
A2. CONSIDERATIONS IN THE INTERPRETATION OF INFRARED SPECTRA OF NONVOLATILE VEHICLES SEPARATED
FROM SOLVENT-TYPE PAINTS
INTRODUCTION
The infrared spectra of vehicles recovered from whole paint are presented in Annex A1. The aim
of this compilation is to aid those using Test Method D 2621 in the practical interpretation of the
spectra they obtain.
The spectra are compiled with one representative spectrum of each vehicle presented in both a prism
and a grating format. In the discussion of the spectra, the general assignment refers to the first
spectrum. The subsequent spectra discussion will include only those bands which aid in the
identification of the particular modifications being illustrated. In addition, some practical information
is provided where it is believed to be helpful to the analyst. In general, previously noted band
assignments are not repeated.
The data compiled here were obtained from spectra prepared on very carefully calibrated
instruments. In comparing them to spectra prepared in any given laboratory, it is expected that the
wavelength values of absorption bands may differ slightly depending upon the calibration of the
instrument used.
GROUP I-ALKYDS
A2.1 Spectrum 1: Ortho-Phthalic Alkyd, Medium Oil absorption bands in this region are due to the C—O—C
Length stretching vibrations of the phthalate ester. These absorptions
−1
are most strongly influenced by the acid portion of the ester
A2.1.1 2.9-μm Region (3448 cm )—The 2.9-μm band in
rather than the alcoholic portion.
alkyds is due to the O—H stretching vibration. This is usually
−1
A2.1.8 13.5 and 14.2-μm Regions (741 and 704 cm )—
attributed to the unesterified hydroxyl OH on the polyhydric
These two bands are due to out-of-plane bending vibrations of
alcohol used in manufacturing the alkyd. This absorption is
ring hydrogens of aromatic compounds having four adjacent
known to increase on drying of unsaturated oil modified alkyds
hydrogens (orthodisubstitution).
due to oxidation of the double bonds. This absorption band can
A2.1.9 Comments:
be used to determine the hydroxyl number of alkyds.
−1 A2.1.9.1 Note that in oil-modified alkyds, the intensity of
A2.1.2 3.3 to 3.6-μm Region (3030 to 2778 cm )—The
−1
the absorption at 8.6 μm (1163 cm ) is indicative of the
bands in this area are all due to aromatic and aliphatic C–H
amount of oil modification or oil length of the alkyd. In
stretching vibrations.
unmodified alkyds, this band may be little more than a side
−1
A2.1.3 5 to 6-μm Region (2000 to 1667 cm )—The
−1
shoulder on the 8.9-μm (1124-cm ) C—O—C absorption. The
5.8-μm band in alkyds is due to the combined C5O stretch of
correlation to oil length is only a very general one in that within
the phthalate and fatty acid esters. Unreacted phthalic anhy-
a given group of alkyds one may say a sample is a “short,”
dride, if present, may be detected by the appearance of a sharp
“medium,” or “long” oil type.
−1
absorption band at approximately 5.6 μm (1786 cm ). Free
A2.1.9.2 Alkyd spectra generally reveal little or no infor-
carboxyl groups (due to unreacted fatty acid or incompletely
mation concerning the type of combined oil or polyol present.
reacted phthalic acid) may often be detected by the appearance
A2.1.9.3 Identification of polyol and unsaponifiables may
of a shoulder on the high wavelength (low frequency) side of
usually be accomplished by infrared examination of saponifi-
the ester carbonyl band.
cation fractions. Identification of the oil acids used usually
−1
A2.1.4 6.2 to 6.4-μm Region (1613 to 1563 cm )—The
requires gas chromatographic analysis of the methylated fatty
doublet appearing in this region of the spectrum is due to
acids recovered by saponification. (For saponification proce-
vibrations associated with the double bonds in an aromatic
dures see Guide D 1467 and Test Method D 1962.)
ring. The band shape and position of this doublet is character-
istic of non-oil modified, o-phthalic alkyds.
A2.2 Spectrum 2: Ortho-Phthalic Alkyd, Long Oil
−1
A2.1.5 6.8 to 6.9-μm Region (1470 to 1449 cm )—This Length
absorption is produced by C–H bending vibrations of methyl- −1
A2.2.1 8.6 μm (1163 cm ); fatty acid ester C—O—C
ene (scissoring deformation) and methyl (asymmetrical defor-
A2.2.2 Comments—Note the difference in the 8.6-μm
−1
mation) groups in the alkyd. The intensity of this absorption
(1163-cm ) peak compared to Spectrum 1, due to increased
band will vary with oil length.
oil length.
−1
A2.1.6 7.2 to 7.3-μm Region (1389 to 1370 cm )—This
A2.3 Spectrum 3: Ortho-Phthalic Alkyd, Tung Oil
absorption band is due to the C—CH symmetrical deforma-
Modified
tion vibration, and is produced by the methyl groups on the
−1
fatty acid chains.
A2.3.1 10.12 μm (988 cm ); –C5C–C5C–C5C– Conju-
−1
A2.1.7 7.5 to 10.0-μm Region (1333 to 1000 cm )—The gated triene unsaturation
D 2621
A2.3.2 Comments—Note the difference in band shapes in A2.8 Spectrum 8: Ortho-Phthalic Alkyd, p-Phenyl Phenol
−1
the 10 to 10.4-μm region (1000 to 962 cm ) compared to Modified
−1
Spectra 1 and 2. Absorption due to conjugated unsaturation (in
A2.8.1 11.4 μm (877 cm ) associated with substituted
such oil types as tung, oiticica, dehydrated castor, and conju-
aromatic rings
−1
gated safflower) occurs here. Oil types used for alkyds 1 and 2
A2.8.2 12.1 μm (826 cm ) associated with substituted
contain only isolated double bonds.
aromatic rings
−1
A2.8.3 13.1 μm (763 cm ) associated with substituted
A2.4 Spectrum 4: Ortho-Isophthalic Alkyd
aromatic rings
−1
−1
A2.4.1 7.8 μm (1282 cm ) isophthalate ester C—O—C A2.8.4 14.4 μm (694 cm ) associated with substituted
−1
A2.4.2 8.2 μm (1220 cm ) isophthalate ester C—O—C
aromatic rings
−1
A2.4.3 8.9 μm (1124 cm ) isophthalate ester C—O—C A2.8.5 Comments—The main identifying band is the
−1 −1
A2.4.4 13.7 μm (730 cm ) meta-disubstituted benzene ring
13.1-μm (763-cm ) band. The other bands are less distinctive,
1−
A2.4.5 Comments—The spectrum of this alkyd is typical of especially the 14.4-μm (694-cm ) area. It is always best to
an isophthalic alkyd. The major band that identifies this as an
consider the positions of the 3 or 4 absorptions in the far end
−1
isophthalate is the 13.7-μm (730-cm ) band. The presence of of the curve as a group in assigning the modifying structure.
orthophthalic alkyd can be suspected by comparison to a
A2.9 Spectrum 9: Ortho-Phthalic Alkyd, Styrene
straight isophthalic alkyd spectrum (see following) and noting
−1 Modified
the influence of the ortho-phthalate at 7.9 μm (1266 cm ), 9.0
−1 −1 −1
μm (1111 cm ), 9.4 μm (1064 cm ), and at 14.2 μm (704 A2.9.1 6.7 μm (1493 cm ) aromatic ring vibration
−1
−1
A2.9.2 13.2 μm (758 cm ) monosubstituted aromatic (5
cm ).
adjacent ring hydrogens)
−1
A2.5 Spectrum 5: Ortho-Phthalic Alkyd, Benzoic Acid A2.9.3 14.3 μm (699 cm ) monosubstituted aromatic (5
Modified adjacent ring hydrogens)
−1
A2.9.4 Comments—The very general forebroadening in the
A2.5.1 14.0 to 14.1 μm (714 to 709 cm ); aromatic ring
−1
13 to 13.3-μm (769 to 758-cm ) area of the ortho substitution
vibration where ring contains five adjacent hydrogens. Position
band is characteristic of styrene modification. The 14.3-μm
is characteristic of benzoate esters.
−1
(699-cm ) absorption that obscures the normally present small
A2.5.2 Comments—The band at approximately 14.0 μm
−1
−1
14.3-μm (699-cm ) band is the primary styrene absorption.
(714 cm ) is the identifying peak for this modification.
−1
−1
Note also the sharp 6.7-μm (1493-cm ) peak which is asso-
Because of the o-disubstitution peak at 14.3μ m (699 cm )
ciated with the presence of an aromatic.
present in
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.