ASTM F2723-08
(Test Method)Standard Test Method for Evaluating Mobile Bearing Knee Tibial Baseplate/Bearing Resistance to Dynamic Disassociation
Standard Test Method for Evaluating Mobile Bearing Knee Tibial Baseplate/Bearing Resistance to Dynamic Disassociation
SIGNIFICANCE AND USE
This test method includes the use of static and fatigue shear and bending force conditions to evaluate the bearing retention mechanism of a mobile bearing knee design and its ability to prevent disassociation.
In general, disassociation does not occur during activities where the contact locations are within the boundaries of the bearing surfaces. Disassociation is most likely to occur with forces at the edges of the bearing component or with large AP shear forces on a posterior stabilized knee tibial component post. Extreme bearing rotation, bone/bearing impingement, severe varus or valgus moments, high flexion or any combination of the above can increase the likelihood of disassociation.
The test method described is applicable to any bicompartmental mobile bearing knee with a bearing retention mechanism. With modification, the test can be applied to a unicompartmental mobile bearing knee with a bearing retention mechanism.
SCOPE
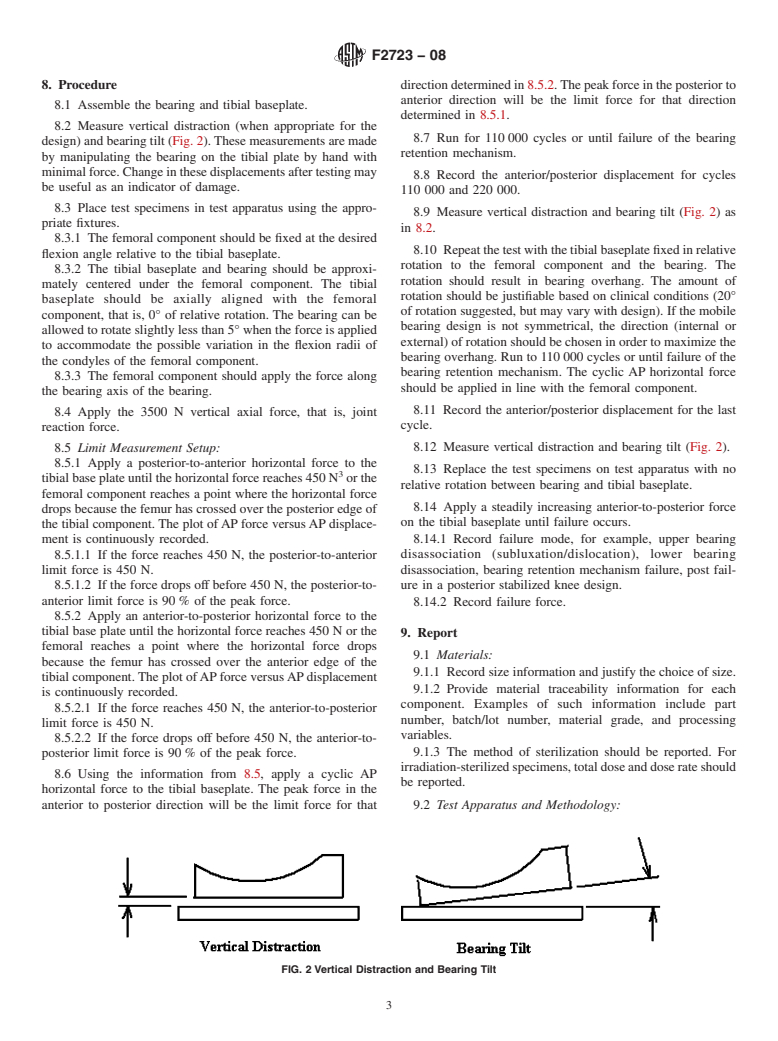
1.1 This test method describes a laboratory method for evaluating the potential for mobile bearing knee tibial baseplate/bearing disassociation under repeated forces.
1.2 The test described is applicable to any bicompartmental mobile bearing knee with a bearing retention mechanism. With modification, the test can be applied to a unicompartmental mobile bearing knee with a bearing retention mechanism.
1.3 Although the methodology described does not replicate all physiological force conditions, it is a means of in vitro comparison of mobile bearing knee designs and the strength of the bearing retention mechanism between the tibial baseplate and bearing components under the stated test conditions.
1.4 The values stated in SI units are regarded as standard.
1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F2723 − 08
StandardTest Method for
Evaluating Mobile Bearing Knee Tibial Baseplate/Bearing
Resistance to Dynamic Disassociation
This standard is issued under the fixed designation F2723; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3.1.3 inferior articulating interfaces—any interface in
which relative motion occurs between the underside of the
1.1 This test method describes a laboratory method for
mobile bearing component and the tibial tray.
evaluating the potential for mobile bearing knee tibial
3.1.4 limiting position—the position of the femoral compo-
baseplate/bearing disassociation under repeated forces.
nent relative to the bearing at which the shear force is at a
1.2 The test described is applicable to any bicompartmental
maximum with anterior-posterior (AP) movement of the femo-
mobile bearing knee with a bearing retention mechanism.With
ral component on the bearing.
modification, the test can be applied to a unicompartmental
3.1.5 mobile bearing—the component between fixed femo-
mobile bearing knee with a bearing retention mechanism.
ral and tibial knee components with an articulating surface on
1.3 Although the methodology described does not replicate
both the inferior and superior sides.
all physiological force conditions, it is a means of in vitro
3.1.6 mobile bearing knee system—a knee prosthesis
comparison of mobile bearing knee designs and the strength of
system, comprised of a tibial component, a mobile bearing
the bearing retention mechanism between the tibial baseplate
component that can rotate or rotate and translate relative to the
and bearing components under the stated test conditions.
tibial component, and a femoral component.
1.4 The values stated in SI units are regarded as standard.
3.1.7 superior articulating interfaces—any interface in
1.5 This standard does not purport to address all of the
whichrelativemotionoccursbetweenthetopsideofthemobile
safety concerns, if any, associated with its use. It is the
bearing component and the femoral bearing component.
responsibility of the user of this standard to establish appro-
3.1.8 tibial baseplate/bearing disassociation—
priate safety and health practices and determine the applica-
unrecoverable physical separation of the bearing and tibial
bility of regulatory limitations prior to use.
baseplate components as a result of bearing distraction or
tilting.
2. Referenced Documents
3.1.9 2-axis orthogonal load frame—a test machine capable
2.1 ASTM Standards:
of applying forces and displacements that act at 90° to each
F1223 Test Method for Determination of Total Knee Re-
other.
placement Constraint
4. Significance and Use
3. Terminology
4.1 This test method includes the use of static and fatigue
3.1 Definitions:
shear and bending force conditions to evaluate the bearing
3.1.1 bearing axis—the line connecting the lowest points on
retention mechanism of a mobile bearing knee design and its
both the lateral and medial condyles of the superior surface of
ability to prevent disassociation.
the mobile bearing.
4.2 In general, disassociation does not occur during activi-
3.1.2 bearing retention mechanism—mechanical means pre-
tieswherethecontactlocationsarewithintheboundariesofthe
venting tibial baseplate/bearing disassociation.
bearing surfaces. Disassociation is most likely to occur with
forces at the edges of the bearing component or with large AP
This test method is under the jurisdiction ofASTM Committee F04 on Medical shear forces on a posterior stabilized knee tibial component
and Surgical Materials and Devices and is the direct responsibility of Subcommittee
post. Extreme bearing rotation, bone/bearing impingement,
F04.22 on Arthroplasty.
severe varus or valgus moments, high flexion or any combi-
Current edition approved June 1, 2008. Published July 2008. DOI: 10.1520/
nation of the above can increase the likelihood of disassocia-
F2723-08.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
tion.
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
4.3 The test method described is applicable to any bicom-
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. partmental mobile bearing knee with a bearing retention
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
F2723 − 08
mechanism. With modification, the test can be applied to a
unicompartmental mobile bearing knee with a bearing reten-
tion mechanism.
5. Apparatus and Materials
5.1 A2-axisorthogonalloadframewithfeedbackcontrolon
bothaxesberequiredfordislocationtesting.Themachinemust
be able to record force and displacement in both axes.
5.1.1 Component Size—Test specimens should be chosen to
maximize the force on the bearing retention mechanism.
Considerations should include bearing thickness (a thicker
bearing would tend to increase the forces on the locking
mechanism, but could also increase the material support for the
locking mechanism), bearing profile/size and tibial baseplate
profile/size (a large bearing on a small tibial baseplate would
tend to increase the overhang with rotation).
5.1.2 Component Quantity—The minimum number of test
samples shall be five.
5.2 Component Configurations—The mobile bearing knee
FIG. 1 Coordinate System and Force Locations
components should be assembled, as they would be for in-vivo
use.
5.2.1 The femoral component flexion angle should be cho-
sen to maximize the forces on the bearing retention mecha-
5.2.6 Oscillating Frequency—The cyclic horizontal force
nism. An engineering analysis may be necessary to determine
shouldbeappliedatafrequencyof0.5to3.0cyclespersecond
the appropriate femoral flexion angle that creates the largest
(0.5 to 3.0 Hz).
shear and/or bending forces on the retention mechanisms. For
5.2.7 Cycle Counter—The test apparatus should be
example, for a gait congruent design, a 0° flexion angle might
equipped with a cycle counter to record the total number of
distribute forces on both the anterior and the posterior sides of
horizontal test cycles.
the locking mechanism, minimizing any bending forces. A
flexion angle of greater than 90° may maximize the posterior
6. Test Specimens
position of the femoral component and consequently increase
6.1 The total knee replacement (TKR) should be the manu-
bending forces on the retention mechanism.
facturer’s designated “standard” or “medium” size unless the
5.2.2 The tibial baseplate should be positioned with the
bearing retention mechanism varies with the size of the knee.
recommended posterior slope. For knee systems where more
If the retention mechanism does vary, an engineering analysis
than one posterior slope is recommended, the largest slope
should be conducted to justify a “worst case.”
should be used.
6.2 The implant shall be in its original packaging as
5.2.3 Component Fixtures—The femoral component is fixed
supplied to the user by the manufacturer.
at the desired flexion angle. The tibial baseplate should be
6.3 If the implant is not available in its package state, the
fixtured with the appropriate posterior slope. The tibial fixtures
condition of the device shall meet all geometry and material
must allow the tibial baseplate to be fixed in relative rotation to
specifications, but may contain slight surface irregularities
the bearing and the femoral components. The test specimen
(that is, “cosmetic rejects”) not considered influential in those
coordinate system is shown in Fig. 1. Fixtures should not
regions of the device deemed critical to the specific test.
inhibit free motion of the bearing, even with substantial
deformation if it should occur.
7. Conditioning
5.2.4 Applied Force—The 3500 N vertical axial force
7.1 Expose the test specimens to a clean atmosphere at a
should be maintained within 63 % for the duration of the test.
temperature of 37 6 2°C for 24 h prior to testing.
The test apparatus or fixtures should allow the force to be
applied through the center of the femoral component (V , Fig.
c 7.2 The test shall be run in a bath at 37 6 2°C that covers
1) to be distributed to the contact points with the tibial
the tibial bearing surface.The bath can be either bovine serum,
component. The peak cyclic horizontal force applied to the
mineral oil, olive oil, or deionized water. Before testing, the
tibial baseplate should be maintained within 63 % for the
implant must be moved cyclically three times in the desired
duration of the test.
direction before data are acquired. These three repetitions can
5.2.5 Displacement Measurement—Displacement sensing
be performed by hand. This procedure is intended to distribute
devices should be capable of measuring the relative motion lubricantbetweenthebearingsurfaceandthetibialcomponent.
between the femoral and tibial baseplate in the anterior- If the bearing is in
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.