ASTM E1173-01(2009)
(Test Method)Standard Test Method for Evaluation of Preoperative, Precatheterization, or Preinjection Skin Preparations
Standard Test Method for Evaluation of Preoperative, Precatheterization, or Preinjection Skin Preparations
SIGNIFICANCE AND USE
These procedures should be used to test topical antimicrobial-containing preparations that are intended to be fast-acting and to reduce significantly the number of organisms on intact skin immediately and, for preoperative and precatheterization preparations, to maintain reductions for an extended time.
SCOPE
1.1 The test method is designed to measure the reduction of the resident microbial flora of the skin.
1.2 A knowledge of microbiological techniques is required for these procedures.
1.3 In this test method, metric units are used for all applications except for linear measure, in which case inches are used, and metric units follow in parentheses.
1.4 This standard does not purport to address all of the safety problems, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
1.5 Performance of this procedure requires a knowledge of regulations pertaining to the protection of human subjects (1).
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: E1173 − 01(Reapproved 2009)
Standard Test Method for
Evaluation of Preoperative, Precatheterization, or
Preinjection Skin Preparations
This standard is issued under the fixed designation E1173; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 3.3 internal reference formulation—a formulation with
demonstrated performance characteristics within a specific
1.1 The test method is designed to measure the reduction of
laboratory.
the resident microbial flora of the skin.
3.4 sampling fluid—a recovery fluid that may or may not
1.2 A knowledge of microbiological techniques is required
containaneutralizertoinactivatetheactiveingredient(s)intest
for these procedures.
and internal reference formulations.
1.3 In this test method, metric units are used for all
3.5 persistence—prolonged or extended antimicrobial activ-
applicationsexceptforlinearmeasure,inwhichcaseinchesare
ity after treatment that prevents or inhibits the proliferation
used, and metric units follow in parentheses.
and/or survival of microorganisms.
1.4 This standard does not purport to address all of the
3.6 neutralization—a process that results in quenching the
safety problems, if any, associated with its use. It is the
antimicrobial activity of a formulation. This may be achieved
responsibility of the user of this standard to establish appro-
through dilution of the formulation to reduce the antimicrobial
priate safety and health practices and determine the applica-
activity, or through use of chemical agents, called neutralizers,
bility of regulatory limitations prior to use.
to curtail antimicrobial activity.
1.5 Performance of this procedure requires a knowledge of
regulations pertaining to the protection of human subjects (1).
4. Summary of Test Method
2. Referenced Documents
4.1 These test methods are conducted on human subjects
selected randomly from a group of volunteers who, after
2.1 ASTM Standards:
refraining voluntarily from using topical and oral antimicrobi-
E1054 Test Methods for Evaluation of Inactivators of Anti-
als for at least two weeks (14 days), exhibit acceptably high
microbial Agents
normal flora counts on the skin sites to be used in testing (see
E1874 Test Method for Recovery of Microorganisms From
Section 8).
Skin using the Cup Scrub Technique
4.2 The antimicrobial activity of the preoperative,
3. Terminology
precatheterization, or preinjection skin preparations is mea-
3.1 active ingredient—a substance added to a formulation sured by comparing microbial counts, obtained at various time
specifically for the inhibition or inactivation of microorgan- intervals after application of a test formulation to skin sites, to
isms. counts obtained from those same sites prior to application of
the test formulation. Skin sites recommended for use in testing
3.2 test formulation—a formulation containing an active
are: 1) the inguinal region and the abdomen for preoperative
ingredient(s).
skin preparations; 2) the inguinal region, the subclavian (clav-
icular) region, and/or the median cubital region of the arm for
precatheterization preparations; and 3) the median cubital
This test method is under the jurisdiction of ASTM Committee E35 on
region of the arm for preinjection skin preparations.
Pesticides, Antimicrobials, and Alternative Control Agents and is the direct
responsibility of Subcommittee E35.15 on Antimicrobial Agents. 4.2.1 Preoperative Skin Preparation—Microbial samples
Current edition approved Oct. 1, 2009. Published November 2009. Originally
are collected from the test sites a minimum of 3 times after
ϵ1
approved in 1987. Last previous edition approved in 2001 as E1173 – 01 . DOI:
treatment application on both moist and dry skin sites. The
10.1520/E1173-01R09.
sample times are 10 min, 30 min, and 6 h (or other appropriate
The boldface numbers in parentheses refer to the list of references at the end of
this standard.
times) post-treatment.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
4.2.2 Precatheterization Preparation—Microbial samples
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
are collected from the test sites a minimum of 3 times after
Standards volume information, refer to the standard’s Document Summary page on
the ASTM website. treatment application on both moist and dry skin sites. The
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
E1173 − 01 (2009)
sample times are “immediate,” 12 h, and 24 h post-treatment. 7.7 Sterile Drape or Dressing —Used to cover treated skin
The immediate sample may be 30 sec to 10 min, depending on sites.
the test material being evaluated.
7.8 Sampling Fluid—Dissolve 0.4 g KH PO , 10.1 g
2 4
4.2.3 Preinjection Preparation—A microbial sample is col-
Na HPO , and 1.0 g isooctylphenoxypolyethoxyethanol in 1 L
2 4
lected from the test site 30 sec post-treatment.
of distilled water. Inactivator(s) specific for the antimicrobial
4.3 The fluid used for sampling the test sites must effec- active(s) in the test and reference formulations may be in-
tively quench (neutralize) the antimicrobial action of all cluded, if appropriate.Adjust to pH 7.8. Dispense in appropri-
formulations tested. The effectiveness of the inactivator must ate volumes and sterilize.
be demonstrated prior to initiation of product-testing, as
7.9 Dilution Fluid—Butterfield’s(2) phosphate-buffered wa-
described in Practices E1054, and using in vivo techniques
ter adjusted to pH 7.2, or other suitable diluent, which may or
consistent with the cup-scrub technique (see Section 10).
may not contain antimicrobial inactivators specific for the test
4.4 To ensure the internal validity of the test, an internal and reference formulations (see Practices E1054).
reference formulation having performance characteristics
7.10 Plating Medium—Soybean-casein digest agar (3), with
known to the laboratory should be tested in parallel with the
or without antimicrobial inactivators.
test formulation.
7.11 Sterile Template Material—Used to demarcate the skin
5. Significance and Use sites; made from paper, plastic, or cloth, for example.
5.1 These procedures should be used to test topical
7.12 Surgical Skin Marker—Used to mark the skin sites.
antimicrobial-containing preparations that are intended to be
NOTE 1—Some markers contain crystal violet, which is inhibitory to
fast-acting and to reduce significantly the number of organisms
many bacteria.
on intact skin immediately and, for preoperative and precath-
eterizationpreparations,tomaintainreductionsforanextended
8. Skin Sites to be Used in Testing
time.
8.1 Preoperative Skin Preparations
8.1.1 The skin sites selected for evaluation of the effective-
6. Apparatus
ness of preoperative skin preparations should include body
6.1 Colony Counter—Any of several types may be used; for
areas that may be surgical sites and should include both moist
example, Quebec colony counters and similar devices, or
and dry skin areas. Bacterial baseline populations should be at
automated, computerized plater/counter systems.
least 3.0 log /cm greater on moist skin sites than the
6.2 Incubator—Any incubator that can maintain a tempera- detection limit of the sampling procedure and at least 2.0 log
ture of 30° 6 2°C may be used.
/cm greater than the detection limit on dry skin sites. High
baseline counts are desired, because variability in the bacterial
6.3 Sterilizer—Any steam sterilizer that can produce the
counts may be reduced. The preferred moist-skin areas are the
conditions of sterilization is acceptable.
inguina, and the preferred dry-skin area is the lower abdomen
6.4 Timer (stopwatch)—One that displays hours, minutes,
below the umbilicus.
and seconds.
8.1.2 Using a 1.5-by-5-in (3.8-by-12.7-cm) sterile template
6.5 Examining Table—Any elevated surface, such as a (for example, paper, plastic, cloth), treatment sites are delin-
3-by-6-ft (0.9-by-1.8-meter) table with mattress or similar eated on the uppermost inner aspects of both thighs (the
padding to allow the subject to recline. inguina), centering the long axis of the template along the
inguinal crease immediately below the groin and marking the
7. Reagents and Materials
corners, using a surgical skin marker. If, due to a subject’s
7.1 Bacteriological Pipettes—10.0 and 2.2-mL or 1.1-mL anatomy, the treatment site cannot be centered along the
capacity, available from most laboratory supply houses. inguinal crease, the site should be positioned on the upper,
inner thigh as close to the crease as possible. In no instance
7.2 Petri Dishes—100 mm by 15 mm, for performing
should sampling be performed on areas not having skin-to-skin
standard plate counts, available from most laboratory supply
contact. The site is then divided on the long axis into
houses.
1-by-1.5-in (2.5-by-3.8-cm) sampling areas, allowing for
7.3 Scrubbing Cups—Autoclavable cylinders, height ap-
spacesofabout0.25in(about0.6cm)betweeneachofthefour
proximately1in(2.5cm),insidediameterofconvenientsizeto
areas.
place on anatomical area to be sampled. Useful diameters
8.1.2.1 Sampling areas are numbered from anterior to pos-
range from approximately 0.5 to 1.5 in (1.3 to 3.8 cm),
terior, beginning with 1 and proceeding perineally to 4. To test
depending on sites to be sampled.
a “worst-case scenario” for efficacy of preoperative skin
7.4 Rubber Policeman or Teflon Scrubbers—Can be fash-
ioned in the laboratory or purchased from most laboratory
The sole source of supply of the apparatus (TELFAnon-adherent dressing, No.
supply houses (whichever type is selected, it should be used
3279) known to the committee at this time is Kendall Co.; Hospital Products;
throughout the course of testing).
Boston, MA 02101. If you are aware of alternative suppliers, please provide this
information to ASTM International Headquarters. Your comments will receive
7.5 Testing Formulation, including directions for use.
careful consideration at a meeting of the responsible technical committee, which
7.6 Sterile Gauge Pads—Used to cover treated skin sites. you may attend.
E1173 − 01 (2009)
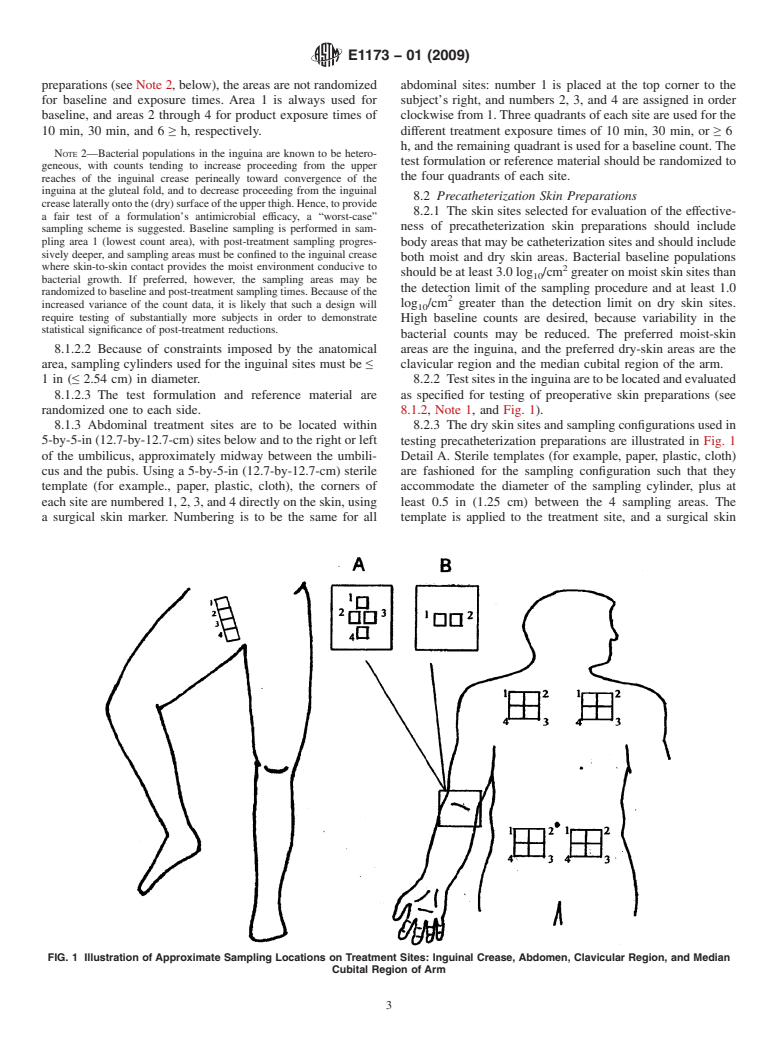
preparations (see Note 2, below), the areas are not randomized abdominal sites: number 1 is placed at the top corner to the
for baseline and exposure times. Area 1 is always used for subject’s right, and numbers 2, 3, and 4 are assigned in order
baseline, and areas 2 through 4 for product exposure times of clockwise from 1.Three quadrants of each site are used for the
10 min, 30 min, and 6 ≥ h, respectively. different treatment exposure times of 10 min, 30 min, or ≥ 6
h, and the remaining quadrant is used for a baseline count. The
NOTE 2—Bacterial populations in the inguina are known to be hetero-
test formulation or reference material should be randomized to
geneous, with counts tending to increase proceeding from the upper
the four quadrants of each site.
reaches of the inguinal crease perineally toward convergence of the
inguina at the gluteal fold, and to decrease proceeding from the inguinal
8.2 Precatheterization Skin Preparations
creaselaterallyontothe(dry)surfaceoftheupperthigh.Hence,toprovide
8.2.1 The skin sites selected for evaluation of the effective-
a fair test of a formulation’s antimicrobial efficacy, a “worst-case”
ness of precatheterization skin preparations should include
sampling scheme is suggested. Baseline sampling is performed in sam-
pling area 1 (lowest count area), with post-treatment sampling progres- body areas that may be catheterization sites and should include
sively deeper, and sampling areas must be confined to the inguinal crease
both moist and dry skin areas. Bacterial baseline populations
where skin-to-skin contact provides the moist environment conducive to 2
should be at least 3.0 log /cm greater on moist skin sites than
bacterial growth. If preferred, however, the sampling areas may be
the detection limit of the sampling procedure and at least 1.0
randomized to baseline and post-treatment sampling times. Because of the
log /cm greater than the detection limit on dry skin sites.
increased variance of the count data, it is likely that such a design will
require testing of substantially more subjects in order to demonstrate
High baseline counts are desired, because variability in the
statistical significance of post-treatment reductions.
bacterial counts may be reduced. The preferred moist-skin
8.1.2.2 Because of constraints imposed by the anatomical
areas are the inguina, and the preferred dry-skin areas are the
area, sampling cylinders used for the inguinal sites must be ≤ clavicular region and the median cubital region of the arm.
1in(≤ 2.54 cm) in diameter. 8.2.2 Testsitesintheinguinaaretobelocatedandevaluated
8.1.2.3 The test formulation and reference material are as specified for testing of preoperative skin preparations (see
randomized one to each side. 8.1.2, Note 1, and Fig. 1).
8.1.3 Abdominal treatment sites are to be located within 8.2.3 The dry skin sites and sampling configurations used in
5-by-5-in (12.7-by-12.7-cm) sites below and to the right or left testing precatheterization preparations are illustrated in Fig. 1
of the umbilicus, approximately midway between the umbili- Detail A. Sterile templates (for example, paper, plastic, cloth)
cus and the pubis. Using a 5-by-5-in (12.7-by-12.7-cm) sterile are fashioned for the sampling configuration such that they
template (for example., paper, plastic, cloth), the corners of accommodate the diameter of the sampling cylinder, plus at
each site are numbered 1, 2, 3, and 4 directly on the skin, using least 0.5 in (1.25 cm) between the 4 sampling areas. The
a surgical skin marker. Numbering is to be the same for all template is applied to the treatment site, and a surgical skin
FIG. 1 Illustration of Approximate Sampling Locations on Treatment Sites: Inguinal Crease, Abdomen, Clavicular Region, and Median
Cubital Region of Arm
E1173 − 01 (2009)
marker is used to demarcate the sampling areas. These are
Z = power of the test = 0.842 for ß = 0.80; and
β
numbered 1 through four at outside corners, beginning at the
D = expected efficacy (expected reduction from baseline).
subject’s upper right and proceeding clockwise in the clavicu-
9.2 Recruit a sufficient number of healthy adult volunteers
lar region, and beginning proximally and proceeding distally
who have no visual evidence of dermatoses, open wounds, or
on
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.