ASTM F1828-97(2014)
(Specification)Standard Specification for Ureteral Stents
Standard Specification for Ureteral Stents
ABSTRACT
This specification covers the chemical, mechanical, and metallurgical requirements for wrought titanium-12 molybdenum- 6 zirconium-2 iron alloy for surgical implants to be used in the manufacture of surgical implants. The heat analysis shall conform to the chemical composition requirements prescribed. Ingot analysis may be used for reporting all chemical requirements, except hydrogen. The wrought titanium-12 molybdenum-6 zirconium-2 iron alloy are classified as bar, forging bar and wire. The ultimate tensile strength, yield strength, elongation, and area reduction of the material shall be tested to meet the requirements prescribed.This specification covers the referee test methods for evaluating the performance characteristics of a single-use ureteral stent with retaining means at both ends, during short term use for drainage of urine from the kidney to the bladder. Ureteral stents shall be tested in accordance with the appropriate biological tests to meet the requirements prescribed. Retention strength, break strength, elongation, dynamic frictional force, and radiopacity shall be tested to meet the requirements prescribed.
SCOPE
1.1 This specification covers the referee test methods for evaluating the performance characteristics of a single-use ureteral stent with retaining means at both ends, during short term use for drainage of urine from the kidney to the bladder. These stents are typically available in diameters of 3.7 Fr to 14.0 Fr, and lengths of 8 cm to 30 cm, and are made of silicone, polyurethane, and other polymers. They are provided non-sterile for sterilization and sterile for single-use.
1.2 Exclusions—Long-term indwelling usage (over 30 days) is encountered with this product, but not commonly, and is therefore considered an exception to this specification. Similarly, the use of ureteral stents for non-ureteral applications such as nephrostomy and ileostomy is excluded from the scope of this specification. Non-sterile ureteral stents are also excluded due to the variability of hospital sterilization equipment and processes and the resulting effects on ureteral stent characteristics.
1.3 The following precautionary statement pertains only to the test method portion, Section 5, of this specification:
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation:F1828 −97 (Reapproved 2014)
Standard Specification for
Ureteral Stents
This standard is issued under the fixed designation F1828; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
INTRODUCTION
The objective of this specification is to describe the test methods used to evaluate the safety and
effectiveness of an indwelling ureteral stent, having retention means at the kidney and bladder ends,
used for urinary drainage of the kidney to the bladder via the ureter.
This specification includes referee test methods that can be used to evaluate the performance
characteristics of ureteral stents. Note that the test methods are not to be construed as production
methods, quality control techniques, or manufacturer’s lot release criteria. The product parameters
addressed by the standard include those determined by the ASTM task group to be pertinent to the
product.
1. Scope 2. Referenced Documents
1.1 This specification covers the referee test methods for 2.1 ASTM Standards:
evaluating the performance characteristics of a single-use D412 Test Methods forVulcanized Rubber andThermoplas-
ureteral stent with retaining means at both ends, during short tic Elastomers—Tension
term use for drainage of urine from the kidney to the bladder. F640 Test Methods for Determining Radiopacity for Medi-
These stents are typically available in diameters of 3.7 Fr to cal Use
14.0Fr,andlengthsof8cmto30cm,andaremadeofsilicone, F748 PracticeforSelectingGenericBiologicalTestMethods
polyurethane, and other polymers. They are provided non- for Materials and Devices
sterile for sterilization and sterile for single-use.
3. Terminology
1.2 Exclusions—Long-termindwellingusage(over30days)
3.1 Definitions of Terms Specific to This Standard:
is encountered with this product, but not commonly, and is
3.1.1 artificial urine—a solution of organic and inorganic
therefore considered an exception to this specification.
compounds that closely simulates the chemical and physical
Similarly, the use of ureteral stents for non-ureteral applica-
properties of normal human urine.Artificial urine will be used
tions such as nephrostomy and ileostomy is excluded from the
asasubstituteforhumanurinetosimulatetheeffectsofhuman
scope of this specification. Non-sterile ureteral stents are also
urine on ureteral stents.
excluded due to the variability of hospital sterilization equip-
ment and processes and the resulting effects on ureteral stent 3.1.2 bladder retention means—physical feature of bladder
end of stent the prevents movement of stent out of bladder.
characteristics.
3.1.3 break strength—peak tensile load required to break
1.3 The following precautionary statement pertains only to
stent.
the test method portion, Section 5, of this specification:
3.1.4 cross section—view of stent tube when cut in a plane
1.4 This standard does not purport to address all of the
perpendicular to length of stent.
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
3.1.5 distal—situated away from the point of origin. The
priate safety and health practices and determine the applica-
distal end of a stent is the end that resides in the bladder, also
bility of regulatory limitations prior to use.
known as the bladder end.
3.1.6 drainage holes—holes in wall of stent tubing that
allow flow of urine into and out of lumen of stent.
This specification is under the jurisdiction of ASTM Committee F04 on
Medical and Surgical Materials and Devices and is the direct responsibility of
Subcommittee F04.34 on Urological Materials and Devices. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved Oct. 1, 2014. Published November 2014. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
approved in 1997. Last previous edition approved in 2006 as F1828 – 97(2006). Standards volume information, refer to the standard’s Document Summary page on
DOI: 10.1520/F1828-97R14. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
F1828−97 (2014)
3.1.7 dynamic frictional force—resistancetorelativemotion The manufacturer need not use this referee test method for
between two surfaces during motion. This force is defined as inspection and quality control.
the coefficient of kinetic friction multiplied by the force acting
3.1.16 retention strength—force required to overcome the
on the surface of the material in a plane perpendicular to the
retaining means on a stent.
surface.
3.1.17 sterility—the state of being free of microorganisms.
3.1.8 elongation—expressed as a percent, is equal to the
For purposes of this specification, sterility is defined as
change in length of a sample of tubing at failure divided by its
freedom from microorganisms when tested according to the
original length. Stretching of the tubing is produced by tensile
methodology defined by the USP for nonparenteral devices.
loading.
3.1.18 tolerances—the allowable deviation from a standard
3.1.9 French size—Scale used to indicate size of tubular
size. The tolerance for the length of a ureteral stent is 60.5 cm
devices, each unit being approximately equal to 0.013 in. or
(0.197 in). the tolerance for the specified French size of a
0.33 mm in diameter.Typical label French sizes are as follows:
ureteral stent is 60.01 mm (0.004 in), or approximately ⁄3
French Size Outside Diameter
French.
in. mm
3.1.19 ureteral stent—an indwelling tubular device that
3.7 0.050,1.23
resides in the kidney, ureter, and bladder containing means for
4.5 0.060,1.50
retaining ends of tube in kidney and bladder.
4.7 0.061,1.57
6.0 0.079,2.00
7.0 0.092,2.33
4. Requirements
8.0 0.105,2.67
8.5 0.112,2.83
4.1 Biocompatibility— Ureteral stents shall be tested in
10.0 0.131,3.33
accordance with the appropriate biological tests contained in
14.0 0.183,4.66
Specification F748 or similar guidance established by the U. S.
3.1.10 kidney retention means—physical feature of kidney
Food and Drug Administration or International Organization
end of stent that prevents movement of stent out of the kidney.
for Standardization (ISO).
3.1.11 length—length of stent is defined as the distance
between the most proximal portion of the bladder retention
5. Special Precautions
means and the most distal portion of the kidney retention
5.1 The following cautionary comments recognize the sen-
means when the stent is laying on a flat surface with the
sitivity of the materials of construction to potential environ-
mainshaft straight. (See Fig. 1).
mental conditions. These are outlined here to point out poten-
3.1.12 lumen—the channel within a tube.
tialsituationsthatcouldadverselyaffecttheperformanceofthe
3.1.13 proximal—situated toward the point of origin. In the
stent during testing.
urinary tract, the kidney is considered to be the point of origin.
5.1.1 Care should be taken during testing and use to prevent
The proximal end of a stent is the end that resides in the renal
damage to the stents. Such damage can be caused by abrasion
pelvis, also known as the kidney end.
and contact with sharp objects or chemical products.
3.1.14 radiopacity—property indicating ability of device to
5.1.2 Stents should be kept away from generators, electric
absorbx-rayenergy,allowingdevicetobeseeninaradiograph
motors,diathermymachines,andfluorescentlightsbecausethe
or fluoroscopic screen.
ozone produced may attack elastomeric materials. This applies
to both storage and handling.
3.1.15 referee test method—the method cited in the pub-
lished specification for the device. This method will be used 5.1.3 To help avoid contamination of the stents proper
when the performance of the ureteral stent is to be evaluated. handling precautions should be observed.
FIG. 1Determine of Stent Length
F1828−97 (2014)
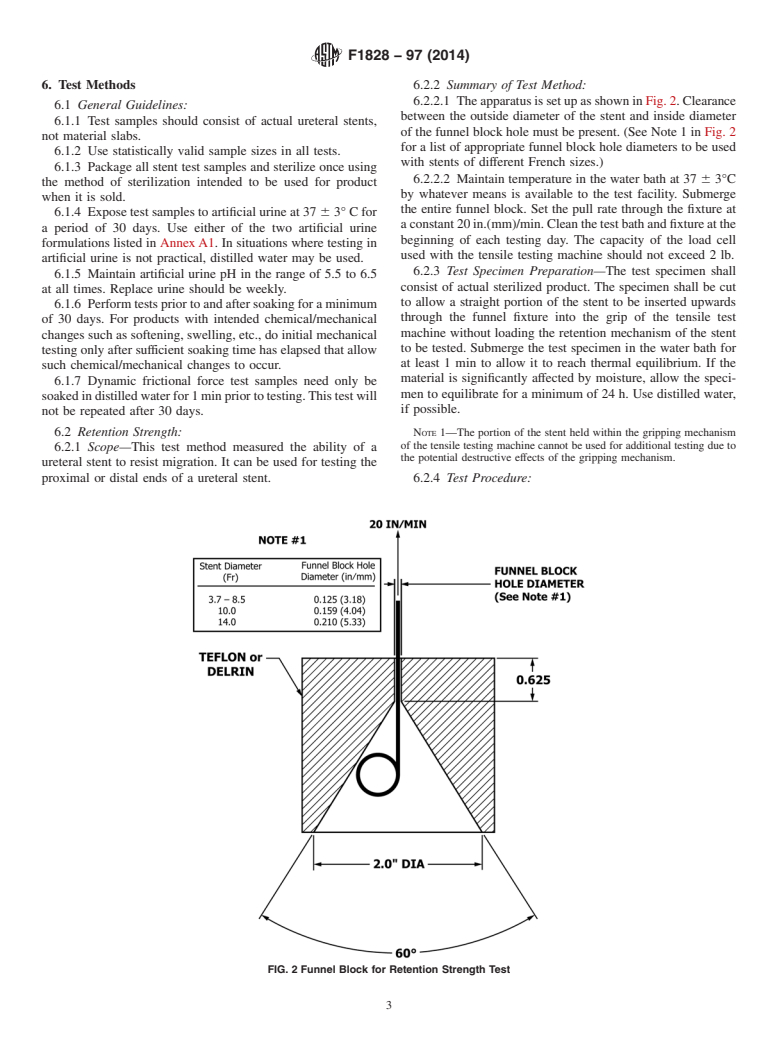
6. Test Methods 6.2.2 Summary of Test Method:
6.2.2.1 TheapparatusissetupasshowninFig.2.Clearance
6.1 General Guidelines:
between the outside diameter of the stent and inside diameter
6.1.1 Test samples should consist of actual ureteral stents,
of the funnel block hole must be present. (See Note 1 in Fig. 2
not material slabs.
for a list of appropriate funnel block hole diameters to be used
6.1.2 Use statistically valid sample sizes in all tests.
with stents of different French sizes.)
6.1.3 Package all stent test samples and sterilize once using
6.2.2.2 Maintain temperature in the water bath at 37 6 3°C
the method of sterilization intended to be used for product
by whatever means is available to the test facility. Submerge
when it is sold.
the entire funnel block. Set the pull rate through the fixture at
6.1.4 Expose test samples to artificial urine at 37 6 3° C for
aconstant20in.(mm)/min.Cleanthetestbathandfixtureatthe
a period of 30 days. Use either of the two artificial urine
beginning of each testing day. The capacity of the load cell
formulations listed in AnnexA1. In situations where testing in
used with the tensile testing machine should not exceed 2 lb.
artificial urine is not practical, distilled water may be used.
6.2.3 Test Specimen Preparation—The test specimen shall
6.1.5 Maintain artificial urine pH in the range of 5.5 to 6.5
consist of actual sterilized product. The specimen shall be cut
at all times. Replace urine should be weekly.
to allow a straight portion of the stent to be inserted upwards
6.1.6 Performtestspriortoandaftersoakingforaminimum
through the funnel fixture into the grip of the tensile test
of 30 days. For products with intended chemical/mechanical
machine without loading the retention mechanism of the stent
changes such as softening, swelling, etc., do initial mechanical
to be tested. Submerge the test specimen in the water bath for
testing only after sufficient soaking time has elapsed that allow
at least 1 min to allow it to reach thermal equilibrium. If the
such chemical/mechanical changes to occur.
material is significantly affected by moisture, allow the speci-
6.1.7 Dynamic frictional force test samples need only be
men to equilibrate for a minimum of 24 h. Use distilled water,
soakedindistilledwaterfor1minpriortotesting.Thistestwill
if possible.
not be repeated after 30 days.
6.2 Retention Strength: NOTE 1—The portion of the stent held within the gripping mechanism
of the tensile testing machine cannot be used for additional testing due to
6.2.1 Scope—This test method measured the ability of a
the potential destructive effects of the gripping mechanism.
ureteral stent to resist migration. It can be used for testing the
proximal or distal ends of a ureteral stent. 6.2.4 Test Procedure:
FIG. 2Funnel Block for Retention Strength Test
------------------
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: F1828 − 97 (Reapproved 2006) F1828 − 97 (Reapproved 2014)
Standard Specification for
Ureteral Stents
This standard is issued under the fixed designation F1828; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
INTRODUCTION
The objective of this specification is to describe the test methods used to evaluate the safety and
effectiveness of an indwelling ureteral stent, having retention means at the kidney and bladder ends,
used for urinary drainage of the kidney to the bladder via the ureter.
This specification includes referee test methods that can be used to evaluate the performance
characteristics of ureteral stents. Note that the test methods are not to be construed as production
methods, quality control techniques, or manufacturer’s lot release criteria. The product parameters
addressed by the standard include those determined by the ASTM task group to be pertinent to the
product.
1. Scope
1.1 This specification covers the referee test methods for evaluating the performance characteristics of a single-use ureteral stent
with retaining means at both ends, during short term use for drainage of urine from the kidney to the bladder. These stents are
typically available in diameters of 3.7 Fr to 14.0 Fr, and lengths of 8 cm to 30 cm, and are made of silicone, polyurethane, and
other polymers. They are provided non-sterile for sterilization and sterile for single-use.
1.2 Exclusions—Long-term indwelling usage (over 30 days) is encountered with this product, but not commonly, and is
therefore considered an exception to this specification. Similarly, the use of ureteral stents for non-ureteral applications such as
nephrostomy and ileostomy is excluded from the scope of this specification. Non-sterile ureteral stents are also excluded due to
the variability of hospital sterilization equipment and processes and the resulting effects on ureteral stent characteristics.
1.3 The following precautionary statement pertains only to the test method portion, Section 5, of this specification:
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2.1 ASTM Standards:
D412 Test Methods for Vulcanized Rubber and Thermoplastic Elastomers—Tension
F640 Test Methods for Determining Radiopacity for Medical Use
F748 Practice for Selecting Generic Biological Test Methods for Materials and Devices
3. Terminology
3.1 Definitions of Terms Specific to This Standard:
3.1.1 artificial urine—a solution of organic and inorganic compounds that closely simulates the chemical and physical properties
of normal human urine. Artificial urine will be used as a substitute for human urine to simulate the effects of human urine on
ureteral stents.
3.1.2 bladder retention means—physical feature of bladder end of stent the prevents movement of stent out of bladder.
This specification is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.34 on Urological Materials and Devices.
Current edition approved March 1, 2006Oct. 1, 2014. Published April 2006November 2014. Originally approved in 1997. Last previous edition approved in 19972006
as F1828 – 97.F1828 – 97(2006). DOI: 10.1520/F1828-97R06.10.1520/F1828-97R14.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
F1828 − 97 (2014)
3.1.3 break strength—peak tensile load required to break stent.
3.1.4 cross section—view of stent tube when cut in a plane perpendicular to length of stent.
3.1.5 distal—situated away from the point of origin. The distal end of a stent is the end that resides in the bladder, also known
as the bladder end.
3.1.6 drainage holes—holes in wall of stent tubing that allow flow of urine into and out of lumen of stent.
3.1.7 dynamic frictional force—resistance to relative motion between two surfaces during motion. This force is defined as the
coefficient of kinetic friction multiplied by the force acting on the surface of the material in a plane perpendicular to the surface.
3.1.8 elongation—expressed as a percent, is equal to the change in length of a sample of tubing at failure divided by its original
length. Stretching of the tubing is produced by tensile loading.
3.1.9 French size—Scale used to indicate size of tubular devices, each unit being approximately equal to 0.013 in. or 0.33 mm
in diameter. Typical label French sizes are as follows:
French Size Outside Diameter
in. mm
3.7 0.050,1.23
4.5 0.060,1.50
4.7 0.061,1.57
6.0 0.079,2.00
7.0 0.092,2.33
8.0 0.105,2.67
8.5 0.112,2.83
10.0 0.131,3.33
14.0 0.183,4.66
3.1.10 kidney retention means—physical feature of kidney end of stent that prevents movement of stent out of the kidney.
3.1.11 length—length of stent is defined as the distance between the most proximal portion of the bladder retention means and
the most distal portion of the kidney retention means when the stent is laying on a flat surface with the mainshaft straight. (See
Fig. 1).
3.1.12 lumen—the channel within a tube.
3.1.13 proximal—situated toward the point of origin. In the urinary tract, the kidney is considered to be the point of origin. The
proximal end of a stent is the end that resides in the renal pelvis, also known as the kidney end.
3.1.14 radiopacity—property indicating ability of device to absorb x-ray energy, allowing device to be seen in a radiograph or
fluoroscopic screen.
3.1.15 referee test method—the method cited in the published specification for the device. This method will be used when the
performance of the ureteral stent is to be evaluated. The manufacturer need not use this referee test method for inspection and
quality control.
3.1.16 retention strength—force required to overcome the retaining means on a stent.
3.1.17 sterility—the state of being free of microorganisms. For purposes of this specification, sterility is defined as freedom from
microorganisms when tested according to the methodology defined by the USP for nonparenteral devices.
3.1.18 tolerances—the allowable deviation from a standard size. The tolerance for the length of a ureteral stent is 60.5 cm
(0.197 in). the tolerance for the specified French size of a ureteral stent is 60.01 mm (0.004 in), or approximately ⁄3 French.
FIG. 1 Determine of Stent Length
F1828 − 97 (2014)
3.1.19 ureteral stent—an indwelling tubular device that resides in the kidney, ureter, and bladder containing means for retaining
ends of tube in kidney and bladder.
4. Requirements
4.1 Biocompatibility— Ureteral stents shall be tested in accordance with the appropriate biological tests contained in
Specification F748 or similar guidance established by the U. S. Food and Drug Administration or International Organization for
Standardization (ISO).
5. Special Precautions
5.1 The following cautionary comments recognize the sensitivity of the materials of construction to potential environmental
conditions. These are outlined here to point out potential situations that could adversely affect the performance of the stent during
testing.
5.1.1 Care should be taken during testing and use to prevent damage to the stents. Such damage can be caused by abrasion and
contact with sharp objects or chemical products.
5.1.2 Stents should be kept away from generators, electric motors, diathermy machines, and fluorescent lights because the ozone
produced may attack elastomeric materials. This applies to both storage and handling.
5.1.3 To help avoid contamination of the stents proper handling precautions should be observed.
6. Test Methods
6.1 General Guidelines:
6.1.1 Test samples should consist of actual ureteral stents, not material slabs.
6.1.2 Use statistically valid sample sizes in all tests.
6.1.3 Package all stent test samples and sterilize once using the method of sterilization intended to be used for product when
it is sold.
6.1.4 Expose test samples to artificial urine at 37 6 3° C for a period of 30 days. Use either of the two artificial urine
formulations listed in Annex A1. In situations where testing in artificial urine is not practical, distilled water may be used.
6.1.5 Maintain artificial urine pH in the range of 5.5 to 6.5 at all times. Replace urine should be weekly.
6.1.6 Perform tests prior to and after soaking for a minimum of 30 days. For products with intended chemical/mechanical
changes such as softening, swelling, etc., do initial mechanical testing only after sufficient soaking time has elapsed that allow such
chemical/mechanical changes to occur.
6.1.7 Dynamic frictional force test samples need only be soaked in distilled water for 1 min prior to testing. This test will not
be repeated after 30 days.
6.2 Retention Strength:
6.2.1 Scope—This test method measured the ability of a ureteral stent to resist migration. It can be used for testing the proximal
or distal ends of a ureteral stent.
6.2.2 Summary of Test Method:
6.2.2.1 The apparatus is set up as shown in Fig. 2. Clearance between the outside diameter of the stent and inside diameter of
the funnel block hole must be present. (See Note 1 in Fig. 2 for a list of appropriate funnel block hole diameters to be used with
stents of different French sizes.)
6.2.2.2 Maintain temperature in the water bath at 37 6 3°C by whatever means is available to the test facility. Submerge the
entire funnel block. Set the pull rate through the fixture at a constant 20 in.(mm)/min. Clean the test bath and fixture at the
beginning of each testing day. The capacity of the load cell used with the tensile testing machine should not exceed 2 lb.
6.2.3 Test Specimen Preparation—The test specimen shall consist of actual sterilized product. The specimen shall be cut to
allow a straight portion of the stent to be inserted upwards through the funnel fixture into the grip of the tensile test machine
without loading the retention mechanism of the stent to be tested. Submerge the test specimen in the water bath for at least 1 min
to allow it to reach thermal equilibrium. If the material is significantly affected by moisture, allow the specimen to equilibrate for
a minimum of 24 h. Use distilled water, if possible.
NOTE 1—The portion of the stent held within the gripping mechanism of the tensile testing machine cannot be used for additional testing due to the
potential destructive effects of the gripping mechanism.
6.2.4 Test Procedure:
6.2.4.1 Ensure test bath is at proper temperature and funnel is submerged. Monitor periodically.
6.2.4.2 When testing a new ureteral stent taken out of its sterile package, (t=0), straighten retention means with appropriate
guidewire. Insert straight portion of stent through bottom of funnel and into grip. When testing at t=30 days, retention means is
not to be straightened prior to testing.
6.2.4.3 Allow stent to reach thermal equilibrium.
6.2.4.4 Pull specimen up through funnel at 20 in./min. Record maximum force required to pull stent completely through funnel.
6.3 Break Strength:
F1828 − 97 (2014)
FIG. 2 Funnel Block for Retention Strength Test
6.3.1 Break strengths of test stents will be determined in accordance with Test Method D412, with the following modifications:
6.3.1.1 Devices used to grip the test specimen in the tensile test machine should be chosen so that the test specimen does not
break at the grip location.
6.3.1.2 Most stents contain drainage holes. Ideally, these stents should break at a drainage hole. This is how tensile failures
typically occur in vivo. However, stents may break in locations other than drainage holes. This type of failure may be indicative
of potential design or process related problems. In stents without drainage holes, this type of failure is to be expected.
6.3.1.3 Only a segment of the test stent is used for the break strength test. The grippers should be separated by 1 in. This 1 in.
segment must contain at least one drainage hole (if drainage holes are present) and should contain the section of the stent with the
smallest cross sectional area or weakest point.
6.4 Elongation—The elongation of stent segments separated by 1 in. between the extensometer grips used to hold the segment
in the tensile test machine or two marks placed on the surface of the stent will be determined in accordance with Test Method D412.
6.5 Dynamic Frictional Force—(Required for Support of Claims of Low Friction):
6.5.1 Scope—This test method measures the dynamic frictional force acting upon the outer surface of ureteral stent during
placement through a small orifice. This test method is intended to simulate passage of a 6 Fr ureteral stent through an endoscope.
6.5.2 Summary of Test Method—Straighten the test stent using a wire mandrel and hydrated in a cylinder of distilled water. Then
place it completely through the appropriate size grommet and into a water column. The mandrel is connected to a load cell that
is pulled at constant rate of 20 in/min. Record the force values measured by the load cell on a chart recorder or minicomputer and
average to determine the dynamic frictional force.
6.5.3 Test Set-Up—(See Fig. 3.)
6.5.3.1 Cylinder—capable of hydrating the full length of the ureteral stent sample.
6.5.3.2 Water Column—used to hydrate the full length of the test stent prior to passage through grommet.
6.5.3.3 Distilled Water—used to hydrate the test stent (and activate any hydrophilic coating present) at room temperature.
6.5.3.4 Grommet—A 0.040 6 0.002 in. thick, 5/8 in. diameter disc, with a 0.079 6 0.002 in. ( 6 mm.) diameter hole in the
center. The grommet is to be made of 55 Shore A Durometer silicone.
6.5.3.5 Grommet Fixture—The grommet is held in place by two aluminum plates containing relief holes approximately ⁄8 in.
larger is diame
...









Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.