ASTM D6420-99(2010)
(Test Method)Standard Test Method for Determination of Gaseous Organic Compounds by Direct Interface Gas Chromatography-Mass Spectrometry
Standard Test Method for Determination of Gaseous Organic Compounds by Direct Interface Gas Chromatography-Mass Spectrometry
SIGNIFICANCE AND USE
This field test method determines the mass concentration of VOHAPs (or any subset) listed in Section 1.
Multiplying the mass concentration by the effluent volumetric flow rate (see 2.2) yields mass emission rates.
This field test method employs the typical laboratory GCMS techniques and QA/QC procedures.
This field test method provides data with accuracy and precision similar to most laboratory GCMS instrumentation.
Note 1—Supporting data are available from ASTM Headquarters Request RR:_______.
SCOPE
1.1 This test method employs a direct interface gas chromatograph/mass spectrometer (GCMS) to identify and quantify the 36 volatile organic compounds (or sub-set of these compounds) listed as follows. The individual Chemical Abstract Service (CAS) numbers are listed after each compound.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D6420 − 99 (Reapproved 2010)
Standard Test Method for
Determination of Gaseous Organic Compounds by Direct
Interface Gas Chromatography-Mass Spectrometry
This standard is issued under the fixed designation D6420; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope ppm(v), using a full scan operation (between 45 and 300
atomic mass units). The range may be extended to higher or
1.1 This test method employs a direct interface gas
lower concentrations using either of the following procedures:
chromatograph/mass spectrometer (GCMS) to identify and
1.4.1 The initial three-point calibration concentrations and
quantify the 36 volatile organic compounds (or sub-set of these
the continuing calibration checks are adjusted to match the
compounds) listed as follows. The individual Chemical Ab-
stack concentrations, or
stract Service (CAS) numbers are listed after each compound.
1.4.2 The three-point calibration is extended to include
Benzene-71432 Methylene chloride-75092
additional concentrations to cover the measurement range.
Bromodichloromethane-75274 1,1,2,2-Tetrachloroethane-79349
Carbon disulfide-75150 1,1,1-Trichloroethane-71556
1.5 The minimum quantification level is 50 % of the lowest
Chloroform-67663 1,1,2-Trichloroethane-79005
calibration concentration. Responses below this level are
Methyl iso-Butyl ketone-108101 p-Xylene-106423
Styrene-100425 Bromomethane-74839
considered to be estimated concentrations, unless a calibration
Tetrachloroethylene-127184 Carbon tetrachloride-56235
standard check is conducted at a lower concentration to
Toluene-108883 Chlorobenzene-108907
demonstrate linearity. The sensitivity of the GCMS measure-
Bromoform-75252 c-1,3-Dichloropropene-10061015
Vinyl acetate-108054 1,2-Dichloroethane-156592
ment system for the individual target analytes depends upon:
Vinyl chloride-75014 1,1-Dichloroethene-75354
1.5.1 The specific instrument response for each target ana-
Chloromethane-74873 t-1,2-Dichloroethene-156605
lyte and the number of mass spectral quantification ions
cis-1,2-Dichloroethene-156592 Methyl ethyl ketone-78933
Dibromochloromethane-124481 2-Hexanone-591786
available.
1,1-Dichloroethane-107062 t-1,3-Dichloropropene-542756
1.5.2 The amount of instrument noise, and
1,2-Dichloropropane-78875 Trichloroethene-79016
1.5.3 The percent moisture content of the sample gas.
Ethylbenzene-100414 m-Xylene-108383
Ethyl chloride-75003 o-Xylene-95476
1.6 This standard does not purport to address all of the
1.2 The test method incorporates a performance-based
safety concerns, if any, associated with its use. It is the
approach, which validates each GCMS analysis by placing
responsibility of the user of this standard to establish appro-
boundaries on the instrument response to gaseous internal
priate safety and health practices and determine the applica-
standards and their specific mass spectral relative abundance.
bility of regulatory limitations prior to use. Additional safety
Using this approach, the test method may be extended to
precautions are described in Section 9.
analyze other compounds.
1.7 This international standard was developed in accor-
dance with internationally recognized principles on standard-
1.3 The test method provides on-site analysis of extracted,
ization established in the Decision on Principles for the
unconditioned, and unsaturated (at the instrument) gas samples
Development of International Standards, Guides and Recom-
from stationary sources. Gas streams with high moisture
mendations issued by the World Trade Organization Technical
content may require conditioning to prevent moisture conden-
Barriers to Trade (TBT) Committee.
sation within the instrument. For these samples, quality assur-
ance (QA) requirements are provided in the test method to
2. Referenced Documents
validate the analysis of polar, water-soluble compounds.
2.1 ASTM Standards:
1.4 Theinstrumentrangeshouldbesufficienttomeasurethe
D1356 Terminology Relating to Sampling and Analysis of
listed volatile organic compounds from 150 ppb(v) to 100
Atmospheres
D3195 Practice for Rotameter Calibration
This test method is under the jurisdiction of ASTM Committee D22 on Air
Quality and is the direct responsibility of Subcommittee D22.03 on Ambient
Atmospheres and Source Emissions. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved Oct. 1, 2010. Published November 2010. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
approved in 1999. Last previous edition approved in 2004 as D6420 – 99 (2004). Standards volume information, refer to the standard’s Document Summary page on
DOI: 10.1520/D6420-99R10. the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D6420 − 99 (2010)
2.2 EPA Test Methods: transported to the GCMS for analysis. Calibration gases are
Method 1–Sample and Velocity Traverses for Stationary introduced at the extractive probe outlet, upstream of the
Sources primaryparticulatefilter.Allsampleextractioncomponentsare
Method 2–Determination of Stack Gas Velocity and Volu- maintained at temperatures that prevent moisture condensation
metric Flow Rate (Type S Pitot Tube) within the measurement system components.
Method 3–Gas Analysis for Carbon Dioxide, Oxygen, Ex-
cess Air, and Dry Molecular Weight
5. Significance and Use
Method 4–Determination of Moisture Content in Stack
5.1 This field test method determines the mass concentra-
Gases
tion of VOHAPs (or any subset) listed in Section 1.
Method 624–Purgables
5.2 Multiplying the mass concentration by the effluent
3. Terminology
volumetric flow rate (see 2.2) yields mass emission rates.
3.1 See Terminology D1356 for definition of terms used in
5.3 This field test method employs the typical laboratory
this test method.
GCMS techniques and QA/QC procedures.
3.2 Definitions of Terms Specific to This Standard:
5.4 This field test method provides data with accuracy and
3.2.1 blank analysis, n—injecting zero air or nitrogen into
precision similar to most laboratory GCMS instrumentation.
the GCMS to determine background levels of the target
NOTE 1—Supporting data are available from ASTM Headquarters
analytes.
Request RR:_______.
3.2.2 CCC, n—continuing calibration check—injecting
calibration gas standards into the GCMS to verify the calibra-
6. Interferences
tion status.
6.1 Analytical Interferences—Analyticalinterferencesoccur
3.2.2.1 Discussion—The continuing calibration check is
when chromatographic peak(s) and quantification ion(s) over-
performed before each testing day, before resuming sampling
lap to such an extent that quantification of specific target
afterinstrumentshutdownormalfunction,andbeforeresuming
compounds is prohibited. The nature of the GCMS technique
sampling after 12 h of continuous instrument operation.
virtually eliminates these types of analytical interferences.
3.2.3 quantification ion, n—a specific ion in the analytes
However, compounds having very simple mass spectra (that is,
mass spectrum that is used for quantification.
only one or two mass fragments) may be difficult to identify
3.2.4 system calibration, n—calibration obtained by inject-
positively.
ing the calibration standard(s) through the entire sampling
6.2 Sampling System Interferences—Sampling system inter-
system.
ferences occur when target analytes are not transported to the
3.2.5 system zero, n—zero obtained by injecting dry nitro-
instrumentation or when compounds damage the measurement
gen or zero gas through the entire sampling system to deter-
system components. Water, reactive particulate matter, adsorp-
mine the system background levels of the target analytes.
tive sites within the sampling system components, and reactive
gases are examples of such potential sampling system interfer-
4. Summary of Test Method
ences. Specific provisions and performance criteria are in-
4.1 Analysis—Volatile Organic Hazardous Air Pollutants
cluded in this test method to detect and prevent the presence of
(VOHAP) are analyzed using gas chromatography (GC) to
sampling system interferences.
separate the individual compounds and mass spectrometry
(MS) to identify the compounds. The MS scans a defined mass
7. Apparatus
range (usually from 45 to 300 atomic mass units (amu) for
7.1 Analytical Instrumentation:
combustion sources) to identify the specific fragments for each
7.1.1 Gas Chromatograph/Mass Spectrometer (GCMS), ca-
molecule. The target analytes are identified positively by: (1)
pable of separating the analyte mixture and detecting com-
comparing eluting analyte GC peak retention times in the total
pounds in the 45 to 300 atomic mass unit (amu) range.
ion chromatograph (TIC) to those contained in a three-point
7.1.2 Personal Computer, with compatible GCMS software
calibration, and (2) examining the mass spectral pattern of the
eluted peaks. Internal standards are used to correct for for control of the GCMS and for data quantification.
hardware-related errors such as different injection volumes,
7.2 Sampling System:
operational temperature fluctuations, and electron multiplier
7.2.1 Sampling Probe, glass, stainless steel, or other appro-
drift.
priate material of sufficient length and physical integrity to
4.2 Sampling—Samples are extracted from the stack or duct
sustainheating,preventadsorptionofanalytes,andtoreachthe
at a constant rate, filtered, conditioned (if required), and
gas sampling point.
7.2.2 Calibration Assembly, typically fabricated by user, to
introduce calibration standards into the sampling system at the
Code of Federal Regulations 40 CFR Part 60, Appendix A, available from
Superintendent of Documents, U.S. Government Printing Office, Washington, DC
probe outlet, upstream of the primary particulate filter, at the
20402.
same pressure and temperature as that of the effluent samples,
Code of Federal Regulations 40 CFR Part 136, Appendix A, available from
with provisions for monitoring the sample pressure and tem-
Superintendent of Documents, U.S. Government Printing Office, Washington, DC
20402. perature during continuing calibrations and effluent sampling.
D6420 − 99 (2010)
7.2.3 Particulate Filters, rated at 0.3 µm, placed immedi- 7.3.4 Tubing, tetrafluorocarbon polymer (or other material),
ately after the heated probe and after the sample condenser of suitable diameter and length to connect cylinder regulators
system. and minimize the adsorption of analytes on the tubing surface.
7.2.4 Pump, leak-free, with heated head, capable of main- 7.3.5 Tubing, 316 stainless steel (or other material), of
taining an adequate sample flow rate (at least 1.5 L/min). suitable diameter and length for heated connections.
7.2.5 Sampling Line, of suitable internal diameter, heated to 7.3.6 Gas Regulators, appropriate for individual gas
prevent sample condensation, made of stainless steel, tetrafluo- cylinders, constructed of materials that minimize adsorption of
rocarbon polymer, or other material that minimizes adsorption analytes.
of analytes, of minimal length.
8. Reagents and Materials
7.2.6 Sample Condenser System,arefrigerationunitcapable
of reducing and removing the moisture of the sample gas to a
8.1 Calibration Gases, gas standards (in nitrogen balance or
level acceptable for sample injection.
other inert gas) for those compounds identified in Section 1,
7.2.7 Sample Flow Rotameters, capable of withstanding
certified by the manufacturer to be accurate to 5 % or better,
sample gas conditions, calibrated in accordance with Practice
used for the initial and continuing calibrations.
D3195.
NOTE 2—The analytical accuracy of the calibration standards must be
7.2.8 Sample Transfer Line, to transport sample from
known. The analytical accuracy for gas mixtures may be concentration
sample interface to GCMS, heated to prevent sample conden-
dependent.
sation and fabricated of stainless steel, tetrafluorocarbon
8.2 Internal Standards, manufacturer-certified mixtures for
polymer, or other material to minimize adsorption of analytes,
co-injection with sample gas.
of minimal length.
8.3 High Purity (HP) Nitrogen or Zero Air, for purging
7.3 Auxiliary Equipment:
sample lines and sampling system components, dilutions, and
7.3.1 Calibration Gas Manifold, capable of delivering ni-
blank runs.
trogen or calibration gases through sampling system or directly
to the instrumentation, with provisions to provide for accurate
9. Hazards
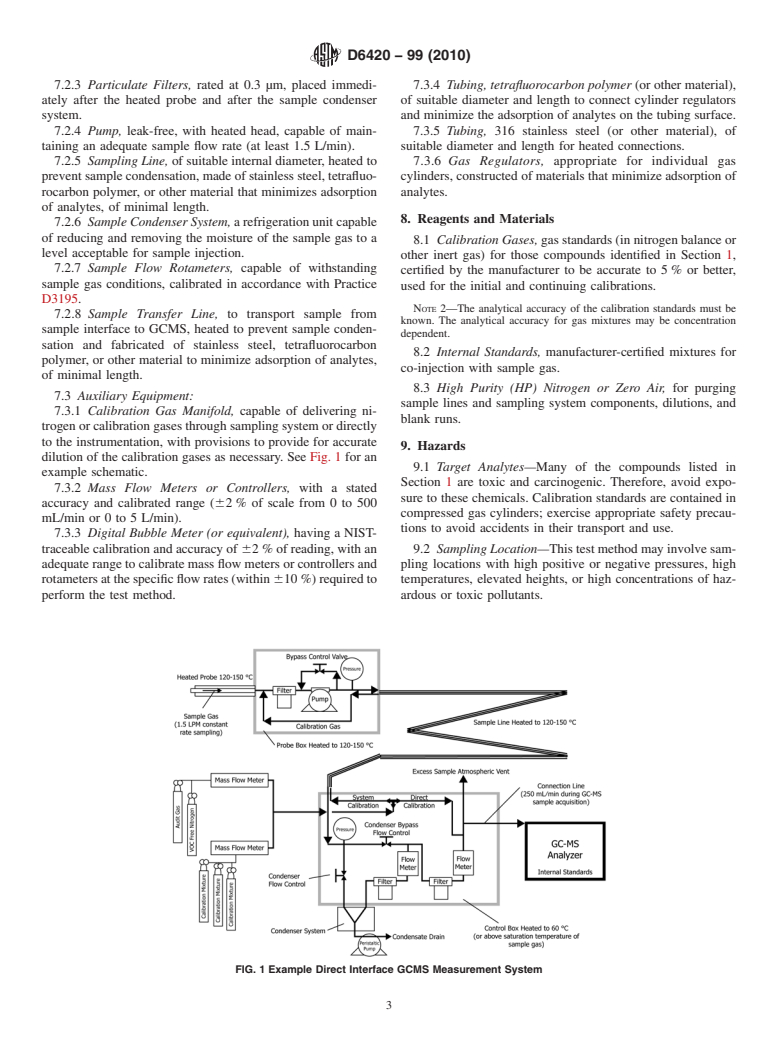
dilution of the calibration gases as necessary. See Fig. 1 for an
9.1 Target Analytes—Many of the compounds listed in
example schematic.
Section 1 are toxic and carcinogenic. Therefore, avoid expo-
7.3.2 Mass Flow Meters or Controllers, with a stated
sure to these chemicals. Calibration standards are contained in
accuracy and calibrated range (62 % of scale from 0 to 500
compressed gas cylinders; exercise appropriate safety precau-
mL/min or 0 to 5 L/min).
tions to avoid accidents in their transport and use.
7.3.3 Digital Bubble Meter (or equivalent), having a NIST-
traceable calibration and accuracy of 62 % of reading, with an 9.2 Sampling Location—This test method may involve sam-
adequate range to calibrate mass flow meters or controllers and pling locations with high positive or negative pressures, high
rotametersatthespecificflowrates(within 610 %)requiredto temperatures, elevated heights, or high concentrations of haz-
perform the test method. ardous or toxic pollutants.
FIG. 1 Example Direct Interface GCMS Measurement System
D6420 − 99 (2010)
TABLE 2 Relative Ion Abundance Criteria for
9.3 Mobile or Remote Laboratory—To avoid exposure to
Bromofluorobenzene
hazardous pollutants and to protect personnel in the laboratory,
Mass Fragment Ion Abundance Criteria
perform a leak check of the sampling system and inspect the
50 15-40 %
sample exhaust equipment before sampling the calibration
75 30-60 %
standards or effluent. Properly vent the exhaust gases.
95 Base peak
96 5-9 % of mass 95
173 <2 % of mass 174
10. Calibration and Standardization
174 >50 % of mass 95
175 5-9 % of mass 174
10.1 Calibration Standards—Becauseoftheincompatibility
176 >95 % but <101 % of mass 174
of some target compounds, many gas blends at each concen-
177 5-9 % of mass 174
tration may be needed to construct a calibration curve for all of
the 36 target analytes listed in 1.1. Obtain or generate calibra-
tion standards of each target compound at nominal con
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.