ASTM G175-03(2011)
(Test Method)Standard Test Method for Evaluating the Ignition Sensitivity and Fault Tolerance of Oxygen Regulators Used for Medical and Emergency Applications
Standard Test Method for Evaluating the Ignition Sensitivity and Fault Tolerance of Oxygen Regulators Used for Medical and Emergency Applications
SIGNIFICANCE AND USE
This test method comprises two phases and is used to evaluate the ignition sensitivity and fault tolerance of oxygen regulators used for medical and emergency applications.
Phase 1: Oxygen Pressure Shock Test—The objective of this test phase is to determine whether the heat from oxygen pressure shocks will result in burnout or visible heat damage to the internal parts of the regulator. Phase 1 is performed according to ISO 10524, Section 11.8.1.
The criteria for an acceptable test are specified in ISO 10524, Section 11.8.1.
The pass/fail criteria for a regulator are specified in ISO 10524, Section 11.8.1.
Phase 2: Regulator Inlet Promoted Ignition Test—The objective of this test phase is to determine if an ignition event upstream of the regulator inlet filter will result in sustained combustion and burnout of the regulator.
The criterion for an acceptable test is either, (1) failure of the regulator, which is defined as the breach of the pressurized regulator component (burnout) and ejection of molten or burning metal or any internal parts from the regulator, or (2) if the regulator does not fail, consumption of at least 90 % of the ignition pill as determined by visual inspection or mass determination. Failure of the regulator at the seal ring does not constitute an acceptable test.
Momentary (less than 1 s) ejection of flame through normal vent paths, with sparks that look similar to those from metal applied to a grinding wheel, is acceptable.
SCOPE
1.1 This standard describes a test method for evaluating the ignition sensitivity and fault tolerance of oxygen regulators used for medical and emergency applications.
1.2 For the purpose of this standard, a pressure regulator is a device, also called a pressure-reducing valve, that is intended for medical or emergency purposes and that is used to convert a medical or emergency gas pressure from a high, variable pressure to a lower, more constant working pressure [21 CFR 868.2700 (a)].
1.3 This standard applies only to oxygen regulators used for medical and emergency applications that are designed and fitted with CGA 540 inlet connections or CGA 870 pin-index adapters (CGA V-1).
1.4 This standard provides an evaluation tool for determining the fault tolerance of oxygen regulators used for medical and emergency applications. A fault tolerant regulator is defined as (1) having a low probability of ignition as evaluated by rapid pressurization testing, and (2) having a low consequence of ignition as evaluated by forced ignition testing.
1.5 This standard is not a design standard; however, it can be used to aid designers in designing and evaluating the safe performance and fault tolerance capability of oxygen regulators used for medical and emergency applications (Guide G128).
Note 1—It is essential that a risk assessment be carried out on breathing gas systems, especially concerning oxygen compatibility (refer to Guides G63 and G94) and toxic product formation due to ignition or decomposition of nonmetallic materials as weighed against the risk of flammability (refer to ISO 15001.2). See Appendix X1 and X2.1 for details.
1.6 This standard is also used to aid those responsible for purchasing or using oxygen regulators used for medical and emergency applications in ensuring that selected regulators are tolerant of the ignition mechanisms that are normally active in oxygen systems.
1.7 This standard does not purport to address the ignition sensitivity and fault tolerance of an oxygen regulator caused by contamination during field maintenance or use. Regulator designers and manufacturers should provide design safeguards to minimize the potential for contamination or its consequences (Guide G88).
1.8 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the...
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: G175 − 03(Reapproved 2011)
Standard Test Method for
Evaluating the Ignition Sensitivity and Fault Tolerance of
Oxygen Regulators Used for Medical and Emergency
Applications
This standard is issued under the fixed designation G175; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope emergency applications in ensuring that selected regulators are
tolerant of the ignition mechanisms that are normally active in
1.1 This standard describes a test method for evaluating the
oxygen systems.
ignition sensitivity and fault tolerance of oxygen regulators
used for medical and emergency applications. 1.7 This standard does not purport to address the ignition
sensitivityandfaulttoleranceofanoxygenregulatorcausedby
1.2 For the purpose of this standard, a pressure regulator is
contamination during field maintenance or use. Regulator
a device, also called a pressure-reducing valve, that is intended
designers and manufacturers should provide design safeguards
for medical or emergency purposes and that is used to convert
tominimizethepotentialforcontaminationoritsconsequences
a medical or emergency gas pressure from a high, variable
(Guide G88).
pressure to a lower, more constant working pressure [21 CFR
1.8 This standard does not purport to address all of the
868.2700 (a)].
safety concerns, if any, associated with its use. It is the
1.3 This standard applies only to oxygen regulators used for
responsibility of the user of this standard to establish appro-
medical and emergency applications that are designed and
priate safety and health practices and determine the applica-
fitted with CGA 540 inlet connections or CGA 870 pin-index
bility of regulatory limitations prior to use.
adapters (CGA V-1).
1.4 This standard provides an evaluation tool for determin-
2. Referenced Documents
ing the fault tolerance of oxygen regulators used for medical
2.1 ASTM Standards:
and emergency applications. A fault tolerant regulator is
G63 Guide for Evaluating Nonmetallic Materials for Oxy-
defined as (1) having a low probability of ignition as evaluated
gen Service
by rapid pressurization testing, and (2) having a low conse-
G88 Guide for Designing Systems for Oxygen Service
quence of ignition as evaluated by forced ignition testing.
G93 Practice for Cleaning Methods and Cleanliness Levels
1.5 This standard is not a design standard; however, it can
for Material and Equipment Used in Oxygen-Enriched
be used to aid designers in designing and evaluating the safe
Environments
performance and fault tolerance capability of oxygen regula-
G94 Guide for Evaluating Metals for Oxygen Service
tors used for medical and emergency applications (Guide
G128 Guide for Control of Hazards and Risks in Oxygen
G128).
Enriched Systems
NOTE 1—It is essential that a risk assessment be carried out on 2.2 ASTM Manual:
breathing gas systems, especially concerning oxygen compatibility (refer
Manual 36 Safe Use of Oxygen and Oxygen Systems
to Guides G63 and G94) and toxic product formation due to ignition or
2.3 Compressed Gas Association (CGA) Standards:
decomposition of nonmetallic materials as weighed against the risk of
CGA E-4 Standard for Gas Pressure Regulators
flammability (refer to ISO 15001.2). See Appendix X1 and X2.1 for
details.
CGA G-4 Oxygen
CGA G-4.1 Cleaning Equipment for Oxygen Service
1.6 This standard is also used to aid those responsible for
CGA V-1 American National/Compressed Gas Association
purchasing or using oxygen regulators used for medical and
1 2
This test method is under the jurisdiction of ASTM Committee G04 on For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Compatibility and Sensitivity of Materials in Oxygen EnrichedAtmospheres and is contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
the direct responsibility of Subcommittee G04.01 on Test Methods. Standards volume information, refer to the standard’s Document Summary page on
Current edition approved April 1, 2011. Published April 2011. Originally the ASTM website.
published as PS 127 – 00. Last published as B175 – 03. DOI: 10.1520/G0175- Available from Compressed Gas Association (CGA), 4221 Walney Rd., 5th
03R11. Floor, Chantilly, VA 20151-2923, http://www.cganet.com.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
G175 − 03 (2011)
Standard for Compressed Gas Cylinder Valve Outlet and 4.3.1 The criterion for an acceptable test is either, (1) failure
Inlet Connections of the regulator, which is defined as the breach of the
pressurized regulator component (burnout) and ejection of
2.4 United States Pharmacopeial Convention Standard:
USP24–NF19 Oxygen monograph molten or burning metal or any internal parts from the
regulator, or (2) if the regulator does not fail, consumption of
2.5 Federal Regulation:
at least 90 % of the ignition pill as determined by visual
21 CFR 868.2700 (a) Pressure regulator
inspectionormassdetermination.Failureoftheregulatoratthe
2.6 ISO Standards:
seal ring does not constitute an acceptable test.
ISO 10524 Pressure regulators and pressure regulators with
4.3.2 Momentary (less than 1 s) ejection of flame through
flow-metering devices for medical gas systems
normal vent paths, with sparks that look similar to those from
ISO 15001 Anaesthetic and respiratory equipment – Com-
metal applied to a grinding wheel, is acceptable.
patibility with oxygen
5. Apparatus
3. Summary of Test Method
5.1 Both phases of this test will be performed in a test
3.1 This test method comprises two phases. A regulator
system as specified by ISO 10524.
must pass both phases in order to be considered ignition
resistant and fault tolerant.
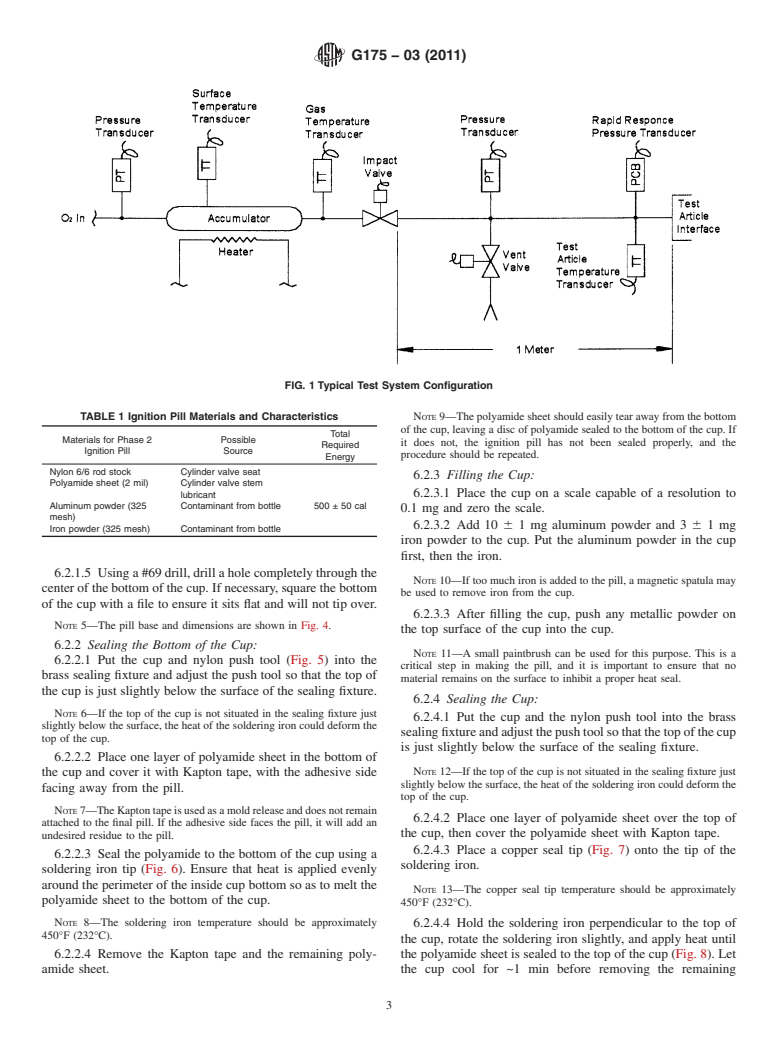
5.2 Fig. 1 depicts a schematic representation of a typical
pneumatic impact test system that complies with ISO 10524.
3.2 Phase 1: Oxygen Pressure Shock Test—In this test
phase, fault tolerance is evaluated by testing the ignition
5.3 Theambienttemperaturesurroundingtheregulatormust
resistance of the regulator design by subjecting the regulator to
be 70 6 9°F (21 6 5°C) for both phases of this test. For Phase
heat from oxygen pressure shocks. The test is performed
2 testing, the test gas temperature can range from 50 to 140°F
according to ISO 10524, Section 11.8.1, which is similar to
(10 to 60°C).
CGA E-4.
6. Materials
3.3 Phase2:RegulatorInletPromotedIgnitionTest—Inthis
test phase, fault tolerance is evaluated by subjecting the 6.1 For both phases of testing, the regulator must be
regulator to the forced application of a positive ignition source
functional and in its normal delivery condition and must be
at the regulator inlet to simulate cylinder valve seat ignition tested as supplied by the manufacturer. If a regulator is
and particle impact events. The ignition source is representa-
supplied with a filter, perform the test with the filter installed.
tive of severe, but realistic, service conditions. The Phase 1 If a prototype or nonproduction unit is used to qualify the
component test system is used for Phase 2 to pressure shock a
design, it must be manufactured using design tolerances,
regulator upstream of its inlet so that an ignition pill is kindled materials, and processes consistent with a production unit. A
to initiate combustion within the regulator.
possible total of eight regulators will be tested; three in Phase
1 and five in Phase 2. If the regulators from Phase 1 are
4. Significance and Use
undamaged, they may be reassembled and used for Phase 2.
4.1 This test method comprises two phases and is used to
6.2 Ignition Pill Manufacture and Assembly—Follow these
evaluate the ignition sensitivity and fault tolerance of oxygen
steps to manufacture and assemble the ignition pill used for
regulators used for medical and emergency applications.
Phase 2 testing. Use the materials listed in Table 1 to
4.2 Phase 1: Oxygen Pressure Shock Test—The objective of manufacture the ignition pills. Total required energy for the
ignition pill is 500 6 50 cal.
this test phase is to determine whether the heat from oxygen
pressure shocks will result in burnout or visible heat damage to
NOTE 2—The ignition pill was developed to simulate both particle
the internal parts of the regulator. Phase 1 is performed
impact events and cylinder valve seat ignition. Particle impact events are
according to ISO 10524, Section 11.8.1. simulated by iron/aluminum powder within the ignition pill. Nonmetallic
promoters within the ignition pill simulate cylinder valve seat ignition.
4.2.1 The criteria for an acceptable test are specified in ISO
The nonmetallic promoters are also used to bind and kindle ignition of the
10524, Section 11.8.1.
metallic powder.
4.2.2 The pass/fail criteria for a regulator are specified in
6.2.1 Forming the Cup:
ISO 10524, Section 11.8.1.
6.2.1.1 Turn the nylon rod down to 0.28 +0/-0.0025 in. OD
4.3 Phase 2: Regulator Inlet Promoted Ignition Test—The
(7.11 +0/-0.064 mm) OD.
objective of this test phase is to determine if an ignition event
6.2.1.2 Place the rod in the brass sealing fixture (Fig. 2),
upstream of the regulator inlet filter will result in sustained
sand the rod face flat, and remove any noticeable burrs.
combustion and burnout of the regulator.
NOTE 3—Fig. 3 shows the nylon rod held in the sealing fixture for
sanding.
Available from U.S. Pharmacopeia (USP), 12601 Twinbrook Pkwy., Rockville,
6.2.1.3 Usea ⁄16in.(4.76mm)diaendmilltoborean~0.06
MD 20852.
in. (1.52 mm) deep cavity in the rod to form a cup.
AvailablefromU.S.GovernmentPrintingOfficeSuperintendentofDocuments,
732 N. Capitol St., NW, Mail Stop: SDE, Washington, DC 20401, http://
6.2.1.4 Cut the cup from the rod.
www.access.gpo.gov.
NOTE4—Thecupshouldbeslightlytallerthan0.13in.(3.30mm).This
Available from International Organization for Standardization (ISO), 1, ch. de
la Voie-Creuse, Case postale 56, CH-1211, Geneva 20, Switzerland, http:// is an initial pill height; the final pill height is achieved after sanding and
www.iso.ch. is based on the required final pill weight.
G175 − 03 (2011)
FIG. 1 Typical Test System Configuration
TABLE 1 Ignition Pill Materials and Characteristics NOTE 9—The polyamide sheet should easily tear away from the bottom
of the cup, leaving a disc of polyamide sealed to the bottom of the cup. If
Total
Materials for Phase 2 Possible
it does not, the ignition pill has not been sealed properly, and the
Required
Ignition Pill Source
procedure should be repeated.
Energy
Nylon 6/6 rod stock Cylinder valve seat
6.2.3 Filling the Cup:
Polyamide sheet (2 mil) Cylinder valve stem
6.2.3.1 Place the cup on a scale capable of a resolution to
lubricant
Aluminum powder (325 Contaminant from bottle 500 ± 50 cal
0.1 mg and zero the scale.
mesh)
6.2.3.2 Add 10 6 1 mg aluminum powder and 3 61mg
Iron powder (325 mesh) Contaminant from bottle
iron powder to the cup. Put the aluminum powder in the cup
first, then the iron.
6.2.1.5 Usinga#69drill,drillaholecompletelythroughthe
NOTE 10—If too much iron is added to the pill, a magnetic spatula may
center of the bottom of the cup. If necessary, square the bottom
be used to remove iron from the cup.
of the cup with a file to ensure it sits flat and will not tip over.
6.2.3.3 After filling the cup, push any metallic powder on
NOTE 5—The pill base and dimensions are shown in Fig. 4.
the top surface of the cup into the cup.
6.2.2 Sealing the Bottom of the Cup:
NOTE 11—A small paintbrush can be used for this purpose. This is a
6.2.2.1 Put the cup and nylon push tool (Fig. 5) into the
critical step in making the pill, and it is important to ensure that no
brass sealing fixture and adjust the push tool so that the top of
material remains on the surface to inhibit a proper heat seal.
the cup is just slightly below the surface of the sealing fixture.
6.2.4 Sealing the Cup:
NOTE 6—If the top of the cup is not situated in the sealing fixture just
6.2.4.1 Put the cup and the nylon push tool into the brass
slightly below the surface, the heat of the soldering iron could deform the
sealingfixtureandadjustthepushtoolsothatthetopofthecup
top of the cup.
is just slightly below the surface of the sealing fixture.
6.2.2.2 Place one layer of polyamide sheet in the bottom of
NOTE 12—If the top of the cup is not situated in the sealing fixture just
the cup and cover it with Kapton tape, with the adhesive side
slightly below the surface, the heat of the soldering iron could deform the
facing away from the pill.
top of the cup.
NOTE7—TheKaptontapeisusedasamoldreleaseanddoesnotremain
6.2.4.2 Place one layer of polyamide sheet over the top of
attached to the final pill. If the adhesive side faces the pill, it will add an
the cup, then cover the polyamide sheet with Kapton tape.
undesired residue to the pill.
6.2.4.3 Place a copper seal tip (Fig. 7) onto the tip of the
6.2.2.3 Seal the polyamide to the bottom of the cup using a
soldering iron.
soldering iron tip (Fig. 6). Ensure that heat is applied evenly
around the perimeter of the inside cup bottom so as to melt the
NOTE 13—The copper seal tip temperature should be approximately
polyamide sheet to the bottom of the cup.
450°F (232°C).
NOTE 8—The soldering iron temperature should be approximately
6.2.4.4 Hold the soldering iron perpendicular to the top of
450°F (232°C).
the cup, rotate the soldering iron slightly, and apply heat until
6.2.2.4 Remove the Kapton tape and the remaining poly- the polyamide sheet is sealed to the top of the cup (Fig. 8). Let
amide sheet. the cup cool for ~1 min before removing the remaining
G175 − 03 (2011)
FIG. 2 Brass Sealing Fixture
FIG. 3 Nylon Rod in Sealing Fixture
polyamide sheet and Kapton tape. Repeat this process until the
cup is capped with five layers of polyamide sheet (Fig. 9).
G175 − 03 (2011)
FIG. 4 Pill Base
NOTE 14—If the cup is sealed properly, a disc of the polyamide sheet
6.4 For Phase 1 testing, the minimum oxygen concentration
will be sealed to it and the remainder of the sheet will easily pull off. It is
shall be of 99.5 % purity and shall not contain more than 10
especiallycriticaltoensurethefirstlayerofpolyamidesheetiscompletely
ppm hydrocarbons. For Phase 2 testing, the minimum oxygen
sealed to the top of the cup, or else the pill contents will leak out and
concentration shall conform to USP24-NF 19, Type 1, or shall
render the pill unusable.
be of 99.0 % purity. Oxygen of higher purity ma
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.