ASTM D2889-95(2010)
(Test Method)Standard Test Method for Calculation of True Vapor Pressures of Petroleum Distillate Fuels
Standard Test Method for Calculation of True Vapor Pressures of Petroleum Distillate Fuels
SIGNIFICANCE AND USE
The true vapor pressure of a distillate fuel is a relative measurement, both of the tendency of the most volatile portion of the fuel to gasify, and of the restraining pressure required to prevent gasification of the most volatile portion. Thus the measurement is of importance when a fuel is to be utilized in applications where no gasification may be tolerated, and temperature-pressure conditions are expected to be near the true vapor pressure of the fuel.
SCOPE
1.1 This test method describes the calculation of true vapor pressures of petroleum distillate fuels for which distillation data may be obtained in accordance with Test Method D86 without reaching a decomposition point prior to obtaining 90 volume % distilled.
1.2 The test method may be used to calculate vapor pressures at temperatures between the 0 % equilibrium flash temperature and the critical temperature of the fuel. Provision is included for obtaining a calculated critical temperature for fuels for which it is not known.
1.3 Critical pressure-temperature data are usually not available for petroleum fuels. However, if both the critical pressure and critical temperature are known, the values shall be used as the coordinates in Fig. 1 to establish a critical point to be used instead of the focal point established as described in 6.5.4; and the calculations described in 6.5 through 6.5.4 are not required. If either a determined true boiling point or determined equilibrium flash vaporization temperature at 0 % distilled at atmospheric pressure is known, the determined value shall be used to establish the lower limit of the bubble-point line referred to in 6.4.
1.4 The method is not reliable for distillate fuels having a boiling range of less than 100°F (38°C) between the Test Method D86 10 and 90 volume % distilled temperatures.
1.5 The values stated in inch-pound units are to be regarded as standard. The values given in parentheses are mathematical conversions to SI units that are provided for information only and are not considered standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D2889 − 95(Reapproved 2010)
Standard Test Method for
Calculation of True Vapor Pressures of Petroleum Distillate
Fuels
This standard is issued under the fixed designation D2889; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
1.1 This test method describes the calculation of true vapor 2.1 ASTM Standards:
pressures of petroleum distillate fuels for which distillation D86Test Method for Distillation of Petroleum Products at
data may be obtained in accordance with Test Method D86 Atmospheric Pressure
without reaching a decomposition point prior to obtaining 90 D287Test Method forAPI Gravity of Crude Petroleum and
volume% distilled. Petroleum Products (Hydrometer Method)
2.2 ASTM Adjuncts:
1.2 The test method may be used to calculate vapor pres-
sures at temperatures between the 0% equilibrium flash Temperature Pressure Conversion Chart (16 by 20–in.
drawings)
temperature and the critical temperature of the fuel. Provision
is included for obtaining a calculated critical temperature for
3. Summary of Test Method
fuels for which it is not known.
1.3 Critical pressure-temperature data are usually not avail- 3.1 Equilibrium flash vaporization (EFV) temperatures are
calculatedfromdistillationdata(TestMethodD86)determined
able for petroleum fuels. However, if both the critical pressure
and critical temperature are known, the values shall be used as on the sample. The distillation data, calculated EFV data, and
APIgravityofthesampleareusedwithagraphicalcorrelation
the coordinates in Fig. 1 to establish a critical point to be used
instead of the focal point established as described in 6.5.4; and procedure to obtain two pairs of temperature-pressure coordi-
natesthroughwhichthebubble-pointlineofthephasediagram
thecalculationsdescribedin6.5through6.5.4arenotrequired.
If either a determined true boiling point or determined equi- for the sample may be drawn. The calculated true vapor
pressure at a specified temperature is obtained by reading the
librium flash vaporization temperature at 0% distilled at
atmospheric pressure is known, the determined value shall be pressure at the intersection of the bubble-point line and
used to establish the lower limit of the bubble-point line specified temperature.
referred to in 6.4.
NOTE1—Detailsoftheprocedureanddatasubstantiatingitsvalidityfor
establishing equilibrium flash vaporization temperatures have been pub-
1.4 The method is not reliable for distillate fuels having a
lished.
boiling range of less than 100°F (38°C) between the Test
Method D86 10 and 90 volume% distilled temperatures.
4. Significance and Use
1.5 The values stated in inch-pound units are to be regarded
4.1 The true vapor pressure of a distillate fuel is a relative
as standard. The values given in parentheses are mathematical
measurement,bothofthetendencyofthemostvolatileportion
conversions to SI units that are provided for information only
of the fuel to gasify, and of the restraining pressure required to
and are not considered standard.
prevent gasification of the most volatile portion. Thus the
1.6 This standard does not purport to address all of the
measurement is of importance when a fuel is to be utilized in
safety concerns, if any, associated with its use. It is the
applications where no gasification may be tolerated, and
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica-
bility of regulatory limitations prior to use.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
This test method is under jurisdiction ofASTM Committee D02 on Petroleum the ASTM website.
Products, Liquid Fuels, and Lubricants and is the direct responsibility of Subcom- Available from ASTM International Headquarters. Order Adjunct No.
mittee D02.04.0K on Correlative Methods. ADJD2889. Original adjunct produced in 1987.
Current edition approved Oct. 1, 2010. Published November 2010. Originally Edmister, W. C., and Okamoto, K. K., “Applied Hydrocarbon
ε1
approved in 1970. Last previous edition approved in 2005 as D2889–95(2005) . Thermodynamics, Part 12: Equilibrium Flash Vaporization Correlations for Petro-
DOI: 10.1520/D2889-95R10. leum Fractions,” Petroleum Refiner, PEREA, Vol 38, No. 8, 1959, p. 117.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D2889 − 95 (2010)
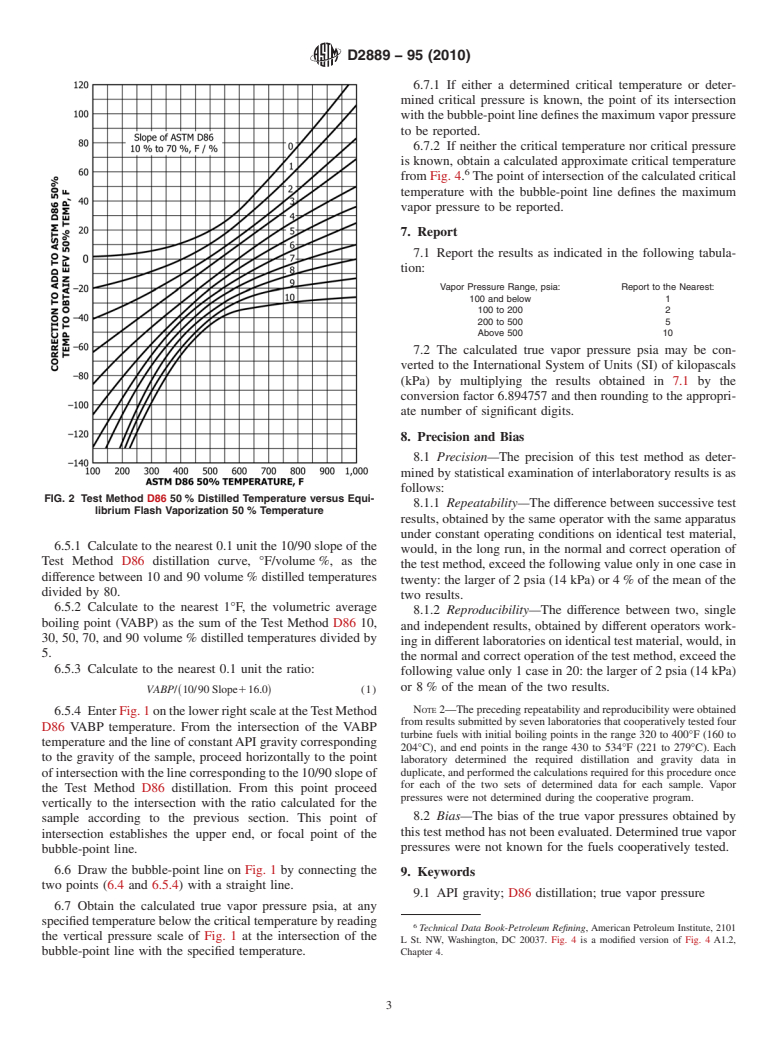
FIG. 1 Test Method D86 Distillation Temperature and Equalization Flash Vaporization Temperature Pressure Conversion Chart
temperature-pressure conditions are expected to be near the 6.3 Calculate the EFV zero volume percent temperature by
true vapor pressure of the fuel. subtracting the sum of the three differences obtained from Fig.
3, from the EFV 50 volume% temperature calculated in
5. Data Requirements
accordance with 6.1.
5.1 Distillation temperatures at the initial boiling point
3,5
6.4 PlotapointonFig.1 atthecoordinates,14.7psiaand
(IBP)and10,30,50,70,and90volume%distilledobtainedin
thecalculatedEFV0%temperature.Thispointestablishesthe
accordance with Test Method D86.
lower end of the phase boundary line commonly referred to as
5.2 APIgravitydeterminedinaccordancewithTestMethod
the bubble-point line. If the EFV 0% temperature at atmo-
D287, or a method of equivalent accuracy.
spheric pressure has been measured, use the measured value
instead of the calculated value.
6. Procedure
6.5 Use the following procedure and the curves on the right
6.1 Calculatethe10/70slope,°F/%,oftheTestMethodD86
portion of Fig. 1 to obtain coordinates for the upper end, or
distillationusingthe10and70volume%distilledtemperature.
focal point, of the bubble-point line. If both the critical
Using this slope and the Test Method D86 50 volume%
distilled temperature, obtain to the nearest 61°F a temperature temperature and critical pressure of the fuel are known, the
difference, °F, from Fig. 2. Add °F to the Method D86 50 calculations described in 6.5.1 through 6.5.4 are not carried
volume% temperature to obtain the equilibrium flash vapor-
out. The critical temperature and critical pressure are used as
ization (EFV) 50 volume% temperature.
the coordinates in Fig. 1 to define a critical point to be used
instead of the focal point.
6.2 Calculate the differences between the Test Method D86
IBPand10volume%,the10and30volume%,andthe30and
50 volume % temperatures. Using these differences, obtain to
the nearest 1°F, the temperature differences between corre- 1
Precision of the test method as given in Section 6 was obtained using 8 ⁄2 by
sponding EFV percentages from Fig. 3. 11–in. charts and should be improved using the 16 by 20–in. charts.
D2889 − 95 (2010)
6.7.1 If either a determined critical temperature or deter-
mined critical pressure is known, the point of its intersection
withthebubble-pointlinedefinesthemaximumvaporpressure
to be reported.
6.7.2 If neither the
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.