ASTM D2889-95(2015)
(Test Method)Standard Test Method for Calculation of True Vapor Pressures of Petroleum Distillate Fuels
Standard Test Method for Calculation of True Vapor Pressures of Petroleum Distillate Fuels
SIGNIFICANCE AND USE
4.1 The true vapor pressure of a distillate fuel is a relative measurement, both of the tendency of the most volatile portion of the fuel to gasify, and of the restraining pressure required to prevent gasification of the most volatile portion. Thus the measurement is of importance when a fuel is to be utilized in applications where no gasification may be tolerated, and temperature-pressure conditions are expected to be near the true vapor pressure of the fuel.
SCOPE
1.1 This test method describes the calculation of true vapor pressures of petroleum distillate fuels for which distillation data may be obtained in accordance with Test Method D86 without reaching a decomposition point prior to obtaining 90 volume % distilled.
1.2 The test method may be used to calculate vapor pressures at temperatures between the 0 % equilibrium flash temperature and the critical temperature of the fuel. Provision is included for obtaining a calculated critical temperature for fuels for which it is not known.
1.3 Critical pressure-temperature data are usually not available for petroleum fuels. However, if both the critical pressure and critical temperature are known, the values shall be used as the coordinates in Fig. 1 to establish a critical point to be used instead of the focal point established as described in 6.5.4; and the calculations described in 6.5 through 6.5.4 are not required. If either a determined true boiling point or determined equilibrium flash vaporization temperature at 0 % distilled at atmospheric pressure is known, the determined value shall be used to establish the lower limit of the bubble-point line referred to in 6.4.
1.4 The method is not reliable for distillate fuels having a boiling range of less than 100 °F (38 °C) between the Test Method D86 10 volume % and 90 volume % distilled temperatures.
1.5 The values stated in inch-pound units are to be regarded as standard. The values given in parentheses are mathematical conversions to SI units that are provided for information only and are not considered standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Buy Standard
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: D2889 − 95 (Reapproved 2015)
Standard Test Method for
Calculation of True Vapor Pressures of Petroleum Distillate
Fuels
This standard is issued under the fixed designation D2889; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2. Referenced Documents
1.1 This test method describes the calculation of true vapor 2.1 ASTM Standards:
pressures of petroleum distillate fuels for which distillation D86Test Method for Distillation of Petroleum Products at
data may be obtained in accordance with Test Method D86 Atmospheric Pressure
without reaching a decomposition point prior to obtaining 90 D287Test Method forAPI Gravity of Crude Petroleum and
volume% distilled. Petroleum Products (Hydrometer Method)
2.2 ASTM Adjuncts:
1.2 The test method may be used to calculate vapor pres-
sures at temperatures between the 0% equilibrium flash TemperaturePressureConversionChart(16by20in.draw-
ings)
temperature and the critical temperature of the fuel. Provision
is included for obtaining a calculated critical temperature for
3. Summary of Test Method
fuels for which it is not known.
1.3 Critical pressure-temperature data are usually not avail- 3.1 Equilibrium flash vaporization (EFV) temperatures are
calculatedfromdistillationdata(TestMethodD86)determined
able for petroleum fuels. However, if both the critical pressure
and critical temperature are known, the values shall be used as on the sample. The distillation data, calculated EFV data, and
APIgravityofthesampleareusedwithagraphicalcorrelation
the coordinates in Fig. 1 to establish a critical point to be used
instead of the focal point established as described in 6.5.4; and procedure to obtain two pairs of temperature-pressure coordi-
natesthroughwhichthebubble-pointlineofthephasediagram
thecalculationsdescribedin6.5through6.5.4arenotrequired.
If either a determined true boiling point or determined equi- for the sample may be drawn. The calculated true vapor
pressure at a specified temperature is obtained by reading the
librium flash vaporization temperature at 0% distilled at
atmospheric pressure is known, the determined value shall be pressure at the intersection of the bubble-point line and
used to establish the lower limit of the bubble-point line specified temperature.
referred to in 6.4.
NOTE1—Detailsoftheprocedureanddatasubstantiatingitsvalidityfor
establishing equilibrium flash vaporization temperatures have been pub-
1.4 The method is not reliable for distillate fuels having a
lished.
boiling range of less than 100°F (38°C) between the Test
Method D86 10 volume % and 90 volume% distilled tem-
4. Significance and Use
peratures.
4.1 The true vapor pressure of a distillate fuel is a relative
1.5 The values stated in inch-pound units are to be regarded
measurement,bothofthetendencyofthemostvolatileportion
as standard. The values given in parentheses are mathematical
of the fuel to gasify, and of the restraining pressure required to
conversions to SI units that are provided for information only
prevent gasification of the most volatile portion. Thus the
and are not considered standard.
measurement is of importance when a fuel is to be utilized in
1.6 This standard does not purport to address all of the
applications where no gasification may be tolerated, and
safety concerns, if any, associated with its use. It is the
responsibility of the user of this standard to establish appro-
priate safety and health practices and determine the applica-
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
bility of regulatory limitations prior to use.
contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
Standards volume information, refer to the standard’s Document Summary page on
This test method is under jurisdiction ofASTM Committee D02 on Petroleum the ASTM website.
Products, Liquid Fuels, and Lubricants and is the direct responsibility of Subcom- Available from ASTM International Headquarters. Order Adjunct No.
mittee D02.04.0K on Correlative Methods. ADJD2889. Original adjunct produced in 1987.
Current edition approved Oct. 1, 2015. Published December 2015. Originally Edmister, W. C., and Okamoto, K. K., “Applied Hydrocarbon
approved in 1970. Last previous edition approved in 2010 as D2889–95 (2010). Thermodynamics, Part 12: Equilibrium Flash Vaporization Correlations for Petro-
DOI: 10.1520/D2889-95R15. leum Fractions,” Petroleum Refiner, PEREA, Vol 38, No. 8, 1959, p. 117.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D2889 − 95 (2015)
FIG. 1 Test Method D86 Distillation Temperature and Equalization Flash Vaporization Temperature Pressure Conversion Chart
temperature-pressure conditions are expected to be near the differences, obtain to the nearest 1°F, the temperature differ-
true vapor pressure of the fuel. ences between corresponding EFV percentages from Fig. 3.
6.3 Calculate the EFV zero volume percent temperature by
5. Data Requirements
subtracting the sum of the three differences obtained from Fig.
5.1 Distillation temperatures at the initial boiling point
3, from the EFV 50 volume% temperature calculated in
(IBP) and 10 volume%, 30 volume%, 50 volume%, 70
accordance with 6.1.
volume%, and 90 volume% distilled obtained in accordance
3,5
6.4 PlotapointonFig.1 atthecoordinates,14.7psiaand
with Test Method D86.
thecalculatedEFV0%temperature.Thispointestablishesthe
5.2 APIgravitydeterminedinaccordancewithTestMethod
lower end of the phase boundary line commonly referred to as
D287, or a method of equivalent accuracy.
the bubble-point line. If the EFV 0% temperature at atmo-
spheric pressure has been measured, use the measured value
6. Procedure
instead of the calculated value.
6.1 Calculatethe10/70slope,°F/%,oftheTestMethodD86
6.5 Use the following procedure and the curves on the right
distillation using the 10volume% and 70 volume% distilled
portion of Fig. 1 to obtain coordinates for the upper end, or
temperature. Using this slope and the Test Method D86 50
focal point, of the bubble-point line. If both the critical
volume% distilled temperature, obtain to the nearest 61°Fa
temperature and critical pressure of the fuel are known, the
temperature difference, °F, from Fig. 2.Add °F to the Method
calculations described in 6.5.1 through 6.5.4 are not carried
D86 50 volume% temperature to obtain the equilibrium flash
out. The critical temperature and critical pressure are used as
vaporization (EFV) 50 volume% temperature.
6.2 Calculate the differences between the Test Method D86
IBPand10volume%,the10volume%and30volume%,and 1
Precision of the test method as given in Section 6 was obtained using 8 ⁄2 by
the 30 volume% and 50 volume % temperatures. Using these 11–in. charts and should be improved using the 16 by 20–in. charts.
D2889 − 95 (2015)
the vertical pressure scale of Fig. 1 at the intersection of the
bubble-point line with the specified temperature.
6.7.1 If either a determined critical temperature or deter-
mined critical pressure is known, the point of its intersection
withthebubble-pointlinedefinesthemaximumvaporpressure
to be report
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: D2889 − 95 (Reapproved 2010) D2889 − 95 (Reapproved 2015)
Standard Test Method for
Calculation of True Vapor Pressures of Petroleum Distillate
Fuels
This standard is issued under the fixed designation D2889; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method describes the calculation of true vapor pressures of petroleum distillate fuels for which distillation data may
be obtained in accordance with Test Method D86 without reaching a decomposition point prior to obtaining 90 volume % distilled.
1.2 The test method may be used to calculate vapor pressures at temperatures between the 0 % equilibrium flash temperature
and the critical temperature of the fuel. Provision is included for obtaining a calculated critical temperature for fuels for which it
is not known.
1.3 Critical pressure-temperature data are usually not available for petroleum fuels. However, if both the critical pressure and
critical temperature are known, the values shall be used as the coordinates in Fig. 1 to establish a critical point to be used instead
of the focal point established as described in 6.5.4; and the calculations described in 6.5 through 6.5.4 are not required. If either
a determined true boiling point or determined equilibrium flash vaporization temperature at 0 % distilled at atmospheric pressure
is known, the determined value shall be used to establish the lower limit of the bubble-point line referred to in 6.4.
1.4 The method is not reliable for distillate fuels having a boiling range of less than 100°F (38°C)100 °F (38 °C) between the
Test Method D86 10 volume % and 90 volume % distilled temperatures.
1.5 The values stated in inch-pound units are to be regarded as standard. The values given in parentheses are mathematical
conversions to SI units that are provided for information only and are not considered standard.
1.6 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility
of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory
limitations prior to use.
2. Referenced Documents
2.1 ASTM Standards:
D86 Test Method for Distillation of Petroleum Products and Liquid Fuels at Atmospheric Pressure
D287 Test Method for API Gravity of Crude Petroleum and Petroleum Products (Hydrometer Method)
2.2 ASTM Adjuncts:
Temperature Pressure Conversion Chart (16 by 20–in.20 in. drawings)
3. Summary of Test Method
3.1 Equilibrium flash vaporization (EFV) temperatures are calculated from distillation data (Test Method D86) determined on
the sample. The distillation data, calculated EFV data, and API gravity of the sample are used with a graphical correlation
procedure to obtain two pairs of temperature-pressure coordinates through which the bubble-point line of the phase diagram for
the sample may be drawn. The calculated true vapor pressure at a specified temperature is obtained by reading the pressure at the
intersection of the bubble-point line and specified temperature.
This test method is under jurisdiction of ASTM Committee D02 on Petroleum Products, Liquid Fuels, and Lubricants and is the direct responsibility of Subcommittee
D02.04.0K on Correlative Methods.
Current edition approved Oct. 1, 2010Oct. 1, 2015. Published November 2010December 2015. Originally approved in 1970. Last previous edition approved in 20052010
ε1
as D2889–95(2005)D2889 – 95 (2010). . DOI: 10.1520/D2889-95R10.10.1520/D2889-95R15.
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Available from ASTM International Headquarters. Order Adjunct No. ADJD2889. Original adjunct produced in 1987.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
D2889 − 95 (2015)
FIG. 1 Test Method D86 Distillation Temperature and Equalization Flash Vaporization Temperature Pressure Conversion Chart
NOTE 1—Details of the procedure and data substantiating its validity for establishing equilibrium flash vaporization temperatures have been published.
4. Significance and Use
4.1 The true vapor pressure of a distillate fuel is a relative measurement, both of the tendency of the most volatile portion of
the fuel to gasify, and of the restraining pressure required to prevent gasification of the most volatile portion. Thus the measurement
is of importance when a fuel is to be utilized in applications where no gasification may be tolerated, and temperature-pressure
conditions are expected to be near the true vapor pressure of the fuel.
5. Data Requirements
5.1 Distillation temperatures at the initial boiling point (IBP) and 10, 30, 50, 70, 10 volume %, 30 volume %, 50 volume %, 70
volume %, and 90 volume % distilled obtained in accordance with Test Method D86.
5.2 API gravity determined in accordance with Test Method D287, or a method of equivalent accuracy.
6. Procedure
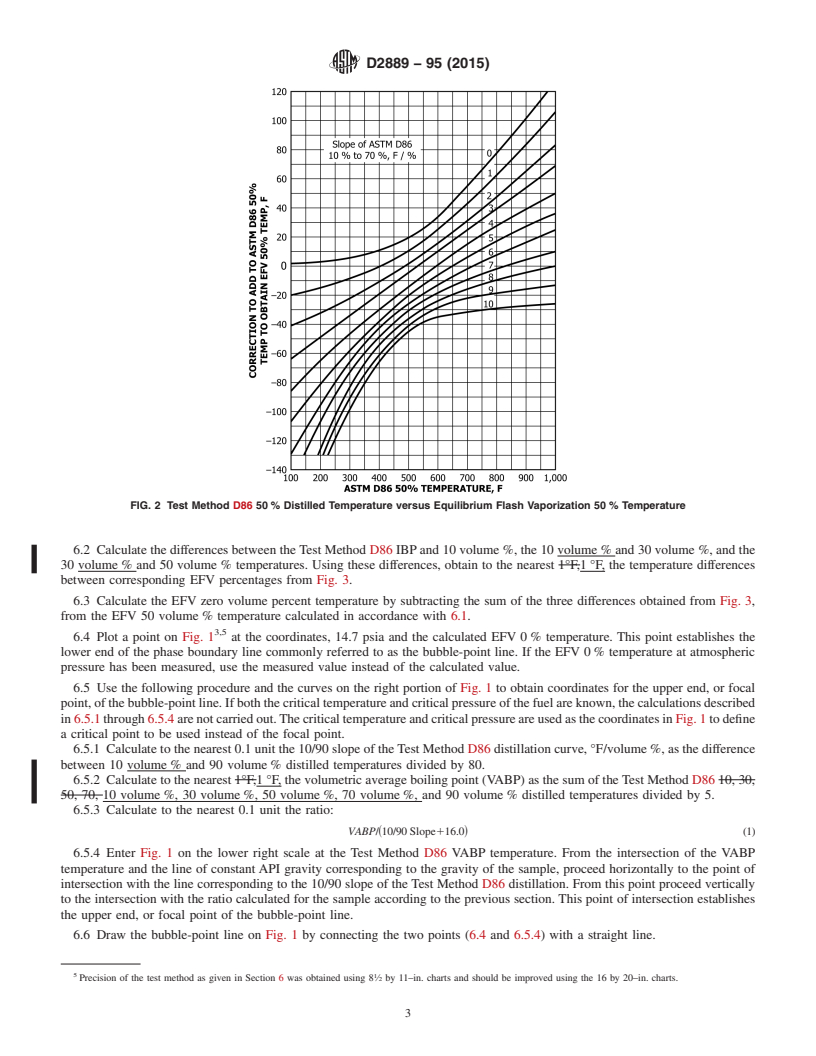
6.1 Calculate the 10/70 slope, °F/%, of the Test Method D86 distillation using the 1010volume % and 70 volume % distilled
temperature. Using this slope and the Test Method D86 50 volume % distilled temperature, obtain to the nearest 61°F61 °F a
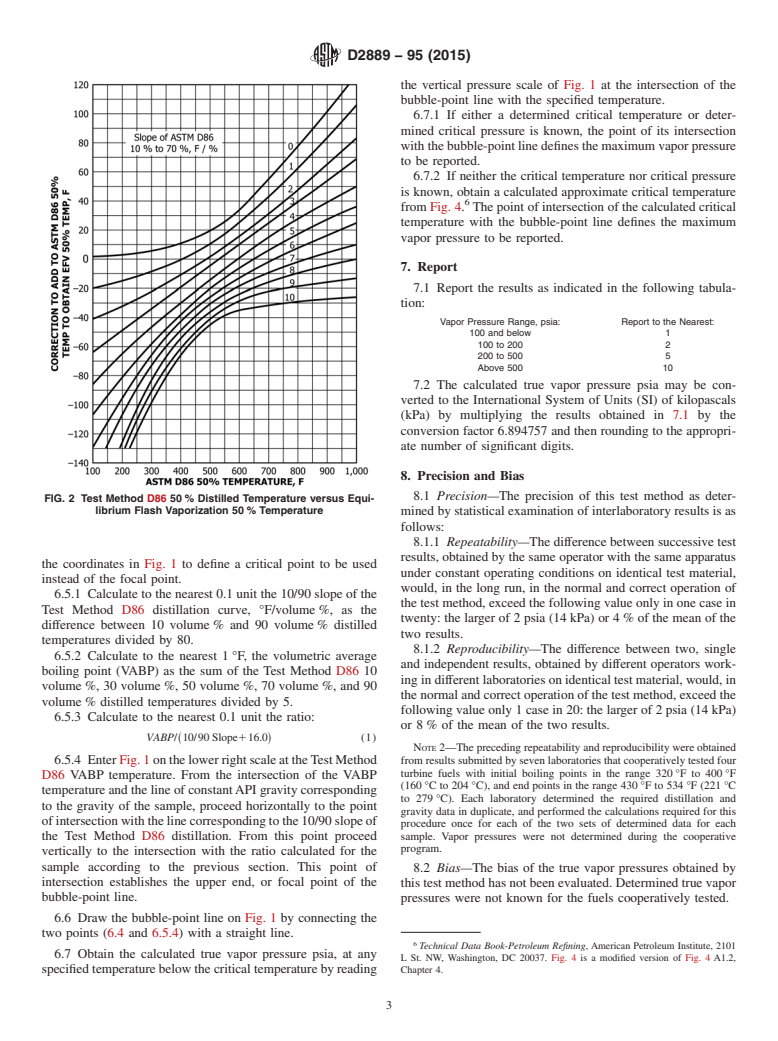
temperature difference, °F, from Fig. 2. Add °F to the Method D86 50 volume % temperature to obtain the equilibrium flash
vaporization (EFV) 50 volume % temperature.
Edmister, W. C., and Okamoto, K. K., “Applied Hydrocarbon Thermodynamics, Part 12: Equilibrium Flash Vaporization Correlations for Petroleum Fractions,”
Petroleum Refiner, PEREA, Vol 38, No. 8, 1959, p. 117.
D2889 − 95 (2015)
FIG. 2 Test Method D86 50 % Distilled Temperature versus Equilibrium Flash Vaporization 50 % Temperature
6.2 Calculate the differences between the Test Method D86 IBP and 10 volume %, the 10 volume % and 30 volume %, and the
30 volume % and 50 volume % temperatures. Using these differences, obtain to the nearest 1°F,1 °F, the temperature differences
between corresponding EFV percentages from Fig. 3.
6.3 Calculate the EFV zero volume percent temperature by subtracting the sum of the three differences obtained from Fig. 3,
from the EFV 50 volume % temperature calculated in accordance with 6.1.
3,5
6.4 Plot a point on Fig. 1 at the coordinates, 14.7 psia and the calculated EFV 0 % temperature. This point establishes the
lower end of the phase boundary line commonly referred to as the bubble-point line. If the EFV 0 % temperature at atmospheric
pressure has been measured, use the measured value instead of the calculated value.
6.5 Use the following procedure and the curves on the right portion of Fig. 1 to obtain coordinates for the upper end, or focal
point, of the bubble-point line. If both the critical temperature and critical pressure of the fuel are known, the calculations described
in 6.5.1 through 6.5.4 are not carried out. The critical temperature and critical pressure are used as the coordinates in Fig. 1 to define
a critical point to be used instead of the focal point.
6.5.1 Calculate to the nearest 0.1 unit the 10/90 slope of the Test Method D86 distillation curve, °F/volume %, as the difference
between 10 volume % and 90 volume % distilled temperature
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.