ASTM F2345-03(2008)
(Test Method)Standard Test Methods for Determination of Static and Cyclic Fatigue Strength of Ceramic Modular Femoral Heads
Standard Test Methods for Determination of Static and Cyclic Fatigue Strength of Ceramic Modular Femoral Heads
SIGNIFICANCE AND USE
These test methods can be used to determine the effects of head and cone materials, design variables, manufacturing, and other conditions on the static and cyclic load-carrying ability of modular femoral heads mounted on the cones of femoral stem prostheses.
These test methods may use actual femoral prostheses or neck-cone models of simplified geometry with the same geometrical and material characteristics as in the implants. In either case, the matching metallic cone region of the test specimen selected shall be of the same material, tolerances, and finishing as the final femoral stem prosthesis.
The static test data may yield valuable information about the relative strengths and merits of different head and cone designs for particular applications. Due to the high forces anticipated for this type of destructive test (>40 kN), the boundary conditions and load levels far exceed possible in vivo loading parameters and therefore may not necessarily be applicable as a quantitative indicator of expected in vivo device performance.
In the fatigue test methods, it is recognized that actual loading in vivo is quite varied, and that no one set of experimental conditions can encompass all possible variations. Thus, the test methods included here represent a simplified model for the purposes of comparisons between designs and materials. These test methods are intended to be performed in air.
The test data may yield valuable information about the relative strengths of different head and cone designs.
SCOPE
1.1 These test methods cover the evaluation of the static and cyclic fatigue strength of ceramic modular femoral heads, mounted on a cone as used on the femoral stem of the total hip arthroplasty.
1.2 These test methods were primarily developed for evaluation of ceramic (Specifications F 603 and F 1873) head designs on metal cones but may have application to other materials.
1.3 The values stated in SI units are to be regarded as standard. No other units of measurement are included in this standard.
1.4 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.
General Information
Relations
Standards Content (Sample)
NOTICE: This standard has either been superseded and replaced by a new version or withdrawn.
Contact ASTM International (www.astm.org) for the latest information
Designation: F2345 − 03(Reapproved 2008)
Standard Test Methods for
Determination of Static and Cyclic Fatigue Strength of
1
Ceramic Modular Femoral Heads
This standard is issued under the fixed designation F2345; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision.Anumber in parentheses indicates the year of last reapproval.A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope 2.2 Other Documents:
DIN 4768Determination of Surface Roughness R,R , and
a z
1.1 Thesetestmethodscovertheevaluationofthestaticand
4
R with Electric Stylus Instruments; Basic Data
max
cyclic fatigue strength of ceramic modular femoral heads,
FDA Guidance Documentfor the Preparation of Premarket
mountedonaconeasusedonthefemoralstemofthetotalhip
NotificationsforCeramicBallHipSystems(draftJan.10,
arthroplasty. 5
1995)
1.2 These test methods were primarily developed for evalu-
3. Terminology
ationofceramic(SpecificationsF603andF1873)headdesigns
3.1 Definitions:
on metal cones but may have application to other materials.
3.1.1 circularity—deviations of taper cross section from a
1.3 The values stated in SI units are to be regarded as
perfect circle.
standard. No other units of measurement are included in this
3.1.2 cone—the proximal end of the femoral component
standard.
fabricated as a truncated right cone and used to engage with a
1.4 This standard does not purport to address all of the
mating conical bore of the modular femoral head.
safety concerns, if any, associated with its use. It is the
3.1.3 cone angle—included angle of cone (Fig. 1).
responsibility of the user of this standard to establish appro-
3.1.4 femoral neck-axis—centerline or axis of symmetry of
priate safety and health practices and determine the applica-
the femoral cone.
bility of regulatory limitations prior to use.
3.1.5 head size—nominal spherical diameter of the head
(generally standardized, but not limited to 22, 26, 28, 32, and
2. Referenced Documents
36 mm for total hips.)
2
2.1 ASTM Standards:
3.1.6 installation load—the force, applied at 0° from femo-
E4Practices for Force Verification of Testing Machines
ral neck axis, used to settle the head on the cone prior to
F603Specification for High-Purity DenseAluminum Oxide
testing.
for Medical Application
3.1.7 load axis—line of action of the compressive force
F1873SpecificationforHigh-PurityDenseYttriaTetragonal
applied to the head.
Zirconium Oxide Polycrystal (Y-TZP) for Surgical Im-
3
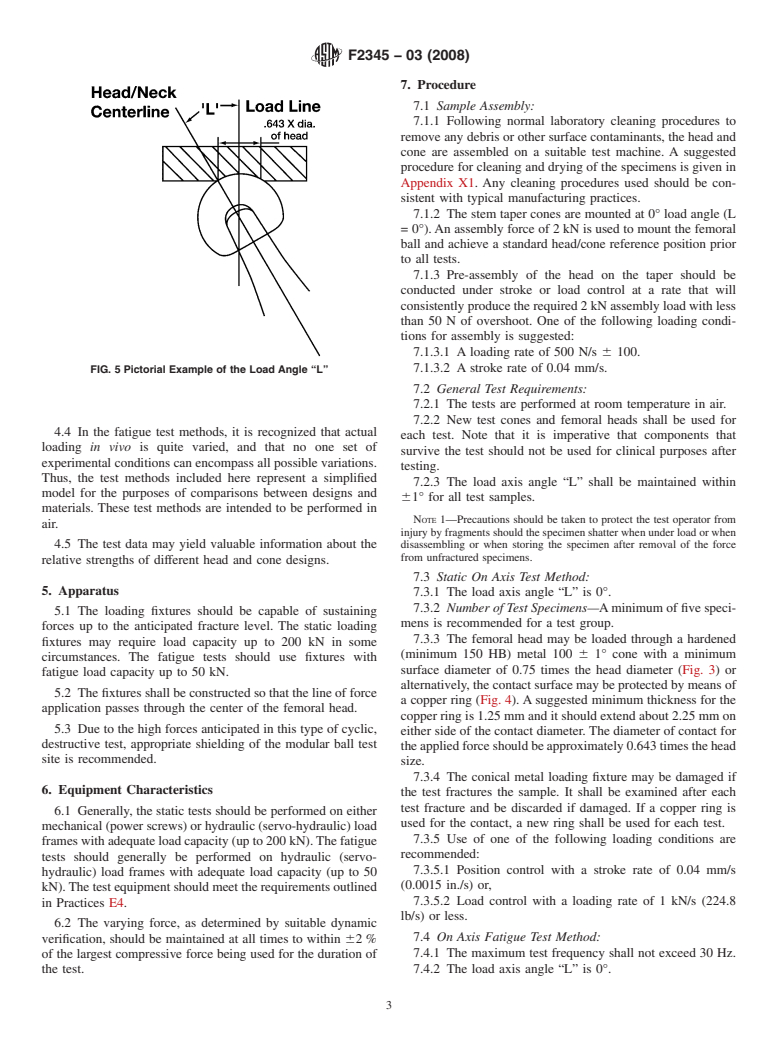
plant Applications (Withdrawn 2007) 3.1.8 load axis angle—the measured angle “L” between the
F1875Practice for Fretting Corrosion Testing of Modular
line of action of the applied force and femoral neck axis (see
Implant Interfaces: Hip Femoral Head-Bore and Cone Fig. 5).
Taper Interface
3.1.9 load magnitude—the peak (absolute value) compres-
sive force of the applied constant amplitude cyclic force.
3.1.10 load rate—rate of applied compressive force.
1
ThistestmethodisunderthejurisdictionofASTMCommitteeF04onMedical
3.1.11 stroke rate—therateofthestrokedisplacementofthe
andSurgicalMaterialsandDevicesandisthedirectresponsibilityofSubcommittee
force applicator.
F04.22 on Arthroplasty.
Current edition approved Dec. 15, 2008. Published December 2008. Originally
3.1.12 surface finish—measured roughness of surface of
approved in 2003. Last previous edition approved in 2003 as F2345–03. DOI:
taper cone or head bore as determined by DIN 4768.
10.1520/F2345-03R08.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or
contactASTM Customer Service at service@astm.org. ForAnnual Book ofASTM
4
Standards volume information, refer to the standard’s Document Summary page on Available from Beuth Verlag GmbH (DIN—DIN Deutsches Institut fur
the ASTM website. Normung e.V.), Burggrafenstrasse 6, 10787, Berlin, Germany.
3 5
The last approved version of this historical standard is referenced on Available from Food and Drug Administration (FDA), 5600 Fishers Ln.,
www.astm.org. Rockville, MD 20857.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2345 − 03 (2008)
FIG. 1 Geometrical Design Criteria for Modular Ball
FIG. 3 Loading in a Metal Cone
FIG. 2 Geometrical Design Criteria for Mating Conical Fit
3.1.13 test frequency—therateofcyclicrepetitionoffatigue
loading in cycles per second.
3.1.14 THR—total hip replacement.
4. Significance and Use
4.1 These test methods can be used to determine the effects
of head and cone materials, design variables, manufacturing,
and other conditions on the static and cyclic load-carrying
ability of modular femoral heads mounted on the cones of
femoral stem prostheses.
4.2 Thesetestmethods
...







Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.